Judge Hacon :
Introduction
- The Claimant ("Coloplast") is the proprietor of European Patent (UK) No. 1 145 729 ("the Patent"). The invention claimed is a ready-to-use urinary catheter assembly. Coloplast alleges that the Patent has been infringed by the Defendant ("MacGregor").
- The Patent expired in September 2017. No injunction is therefore in issue but Coloplast seeks damages for infringement before the date of expiry. As part of its defence MacGregor counterclaims for a declaration that the Patent was invalid.
- Stuart Baran appeared for Coloplast, Thomas Mitcheson QC and Tim Austen for MacGregor.
The Patent
- Urinary catheters were well known at the filing date, 18 September 1997. The Patent claimed two earlier priority dates but these were not relied on by Coloplast.
- Catheters are inserted into the patient's urethra to assist in the emptying of the bladder. An important feature is its ability to slide as easily as is possible through the urethra giving minimum discomfort and without damaging the urethra walls. It was known to coat the relevant part of the catheter with a hydrophilic surface layer. Immediately prior to use this layer was exposed to what the Patent calls 'a liquid swelling medium', causing the layer to expand and impart a very slippery character to the catheter and thus facilitate insertion. Typically the liquid swelling medium is water. For brevity I will hereafter refer just to water, save in the case of one item of prior art in which the liquid swelling medium is a drug solution.
- Urinary catheters were divided into two relevant types. The first, indwelling catheters, consists of catheters which remain in the urethra for a prolonged period, possibly some weeks. They are used for patients who are infirm or who are undergoing either surgery or other lengthy treatment in hospital. They are administered by nursing staff and require a means to fasten the catheter securely in place for the period required.
- The second type are 'intermittent catheters'. These are used for a one-off insertion to empty the bladder, generally several times a day, after which they are removed and very often discarded without reuse. Intermittent catheters are usually administered by the patient themselves at home or elsewhere outside a hospital. Ease of insertion and removal is therefore of particular importance for intermittent catheters.
- The specification points out that a user may find it difficult to find a source of water of a sufficiently aseptic standard to activate the hydrophilic coating. The solution is to store the catheter in packaging along with sterile water.
- The packaging of the assembly is 'gas-impermeable' which, as the Patent explains, means that it should prevent evaporation of the water for a period exceeding the shelf life of the catheter assembly.
- Claims 1, 3 and 5 were in issue. I set them out here without reference numerals and with the elements of the claims numbered in the manner used by the parties:
1. 1.1 A urinary catheter assembly
1.2 comprising at least one urinary catheter,
1.3 the catheter having a catheter tube
1.4 coated on its external surface on a substantial part of its length from its distal end with a hydrophilic surface layer in the form of a hydrophilic coating
1.5 intended to produce a low-friction surface character of the catheter by treatment with a liquid swelling medium prior to use of the catheter
1.6 and a catheter package
1.7 made of a gas impermeable material formed by a multiple layer thermoplastic film material comprising aluminium,
1.8 the package having a cavity for accommodation of the catheter,
1.9 wherein the cavity accommodates said liquid swelling medium for provision of a ready-to-use catheter assembly.
3. 3.1 A urinary catheter assembly as claimed in claim 2 [which requires the whole of the catheter package to be made of gas impermeable material], characterized in that
3.2 the catheter package is formed from two sheets of the gas impermeable film material connected with each other by a gas impermeable joint
3.3 defining the cavity for accommodating the catheter and the liquid swelling medium.
5. 5.1 A urinary catheter assembly as claimed in claim 3 or 4, characterized in that
5.2 said joint is arranged [to] provide said cavity with a cross-section narrowly surrounding the catheter.
The issues
- The broad issues were:
(1) Infringement, which turned on a point of construction.
(2) Novelty over either of two items of prior art:
(a) PCT Application no. WO 96/30277 ("Rødsten"), and
(b) PCT Application no. WO 97/26937 ("Israelsson").
(3) Inventive step over the prior art relied on for lack of novelty and one further citation:
(c) Japanese patent no. JP-A-55-012265 ("Shibatani")
(4) Insufficiency.
The person skilled in the art
- Mostly there was agreement about the skilled team. It would be led by a developer of catheters. Such an individual would be assisted by a clinician who would inform the developer about the needs of patients.
- The disagreement concerned whether the developer would have had knowledge and experience of indwelling catheters as well as intermittent catheters.
- The addressees of a patent specification are
"those likely to have a practical interest in the subject matter of [the] invention",
such individuals being
"persons with practical knowledge and experience of the kind of work in which the invention was intended to be used"
per Lord Diplock in Catnic Components Ltd v Hill & Smith Ltd [1982] R.P.C. 183, at p.242-3.
- The skilled team has an interest in, together with knowledge and experience of the invention as claimed. This raised the question whether the invention here claimed extended to indwelling catheters. If so, necessarily the skilled team would be familiar with them.
- Dr Baran submitted in his skeleton argument that the claims covered only intermittent catheters. The trial went ahead on an unspoken basis that these were what mattered most, which is possibly why Dr Baran did not develop reasons for the limited scope of the claims as a formal matter of construction. MacGregor did not raise this as a point of construction either, possibly because it had reasons not to argue for a broad scope. Nonetheless, I must deal with it.
- The effect of Coloplast's case was that the word 'catheter' in the claims should be construed to mean 'intermittent catheter'. Yet it was not in doubt that the addressee would have known that catheters come in two varieties and the words of the claims did not specify just one of them. Of course, claims must be construed in the context of the specification as a whole. Had there been, for instance, a definition of 'catheter' in the specification, that would have been decisive. There was none. Moreover, this is not an instance in which a term of the claims is ambiguous. It would take a strong indication in the specification for the addressee to understand that 'catheter' in the claims meant only one type of catheter.
- Paragraph [0002] of the specification makes an early statement as to the catheters covered by the invention:
"Urinary catheters of the kind to which the invention pertains are known, inter alia from [14 identified items of prior art]."
It was not suggested by Coloplast that all 14 disclosed only intermittent catheters. If they do not, this would severely undermine the contention that the scope of claims 1, 3 and 5 is limited to intermittent catheters.
- There is no doubt that the problem said to be overcome by the invention related to intermittent catheters. Paragraph [0004] states:
"[0004] An important feature of any urinary catheter used for intermittent catheterisation of the bladder of an incontinent user is the ability of the catheter to slide easily through the urethra without exposing the urethral walls to any risk of damage. Catheters of the kind to which the inventions pertains [sic] have been developed to meet this need by imparting an extremely low friction character to at least part of the surface of the catheter which is actually introduced into the urethra. …"
- The reader is here informed that catheters of the invention meet a need arising in the context of intermittent catheterisation. But that is not a statement limiting their use exclusively to intermittent catheterisation. Similarly, paragraph [0006] tells the reader that the catheters as claimed have particular advantages when administered by the patient, but does not limit the potential application of such catheters:
"[0006] When catheters of this kind are used directly by end users outside the medical environment of a hospital or a clinic, e.g. by or [sic] tetraplegic patients who often have a very poor dexterity, and therefore need a very simple insertion procedure, the most common liquid swelling medium used for preparation of the catheter immediately prior to use would be normal tap water."
- The specification goes on to say that the tap water available, e.g. in public toilets, may not be of a sufficiently clean standard. Hence the advantage of having a ready-wetted hydrophilic layer or alternatively a means of wetting the layer using water contained within the catheter packaging. Again, this does not imply that the catheter assemblies claimed exclude indwelling catheters used in a hospital.
- The closest that the specification comes to indicating a narrow construction of 'catheter' is at paragraph [0008]:
"[0008] On this background, it is the object of the invention to improve and facilitate the performance of the intermittent urinary catheterisation in any type of environment by providing a ready to use urinary catheter assembly comprising a catheter which can be withdrawn from its package and is prepared for direct insertion in the urethra and in a substantially sterile condition, whereby the general quality of life for users of intermittent catheterisation would be greatly improved."
- 'On this background' indicates that what is being said in this paragraph follows from what was said before: the advantage conferred by the invention will be felt by users of intermittent catheters. That is not the same as saying that the invention cannot be used for indwelling administration. None of the evidence suggested a technical reason why the invention could not apply to indwelling catheters.
- I therefore take the view that the term 'catheter' in claims 1, 3 and 5 should be given its usual meaning and that those claims encompass assemblies comprising catheters for both intermittent and indwelling administration.
- The team referred to above was therefore skilled in both types of catheter and included a developer and clinician familiar with each of them.
The witnesses
- Both parties filed evidence from two experts, a developer of intermittent catheters and a clinician with experience of using them. Both also had a witness of fact. In the case of MacGregor this was Michael Sorth, a director of MacGregor. Mr Sorth did not provide a witness statement but he had signed MacGregor's Reply to Defence to Counterclaim. As is usual in this court, at the case management conference it was ordered that the pleadings would stand as evidence in chief of the facts stated in them. Mr Sorth was cross-examined.
- Coloplast's developer was Gunvor Anette Israelsson, a former employee of Astra Tech ("Astra"), part of the Astra Zeneca group. In September 1997 Astra was the biggest player in the urinary catheter market, followed by Coloplast. At that time the most used intermittent catheter in Europe, and possibly beyond, was an Astra product called 'LoFric'. It had been launched in 1983 and was the first urinary catheter to have a hydrophilic coating. LoFric is still on the market today. Unlike the patented product, water from a source outside the packaging must be added to the hydrophilic coating before use.
- Ms Israelsson started at Astra in 1991, joining a team working on intermittent catheter development. She is the first-named inventor on the Israelsson prior art.
- Coloplast's clinician was Kathy Howard, a nurse with experience of some 45 years working with urology patients using intermittent catheters.
- Jonathan Carr was Coloplast's witness of fact, his evidence being directed to the commercial success of Coloplast's 'SpeediCath' product, the intermittent catheter marketed by Coloplast which embodied the invention claimed in the Patent. Mr Carr has worked in finance and accounting roles at Coloplast since 1989 and is now Senior Commercial Excellence Manager.
- Jan Torstensen was the developer who gave expert evidence on behalf of MacGregor, although most of his experience came from his time at Coloplast where he was employed from 1991 to 2012. Even more unusually, he is a named inventor on the Patent, giving evidence in support of MacGregor's contention that the Patent is invalid. Mr Torstensen is currently Senior Product Engineer at Dansac A/S, a manufacturer of ostomy products.
- Ann Cahill gave clinical expert evidence for MacGregor. At the Patent's filing date she was a nurse in Hertfordshire specialising in continence issues. Ms Cahill has also contributed to a book, published in 1994, about intermittent catheterisation. She retired in 2011.
- In my view all the witnesses were doing their honest best to help the court and in the cases of Mr Carr and Mr Sorth, to state the facts accurately as they saw them.
The common general knowledge
- There were two issues of dispute between the parties about the common general knowledge.
- The first I have already referred to: whether the skilled team would have been familiar with indwelling catheters. I have decided that the answer is yes because the claims cover indwelling catheters. However, since the parties did not address the scope of the claim in the sense of whether it covers indwelling catheters, I will also consider the evidence of what would have been known to a developer of intermittent catheters.
- Two such developers gave evidence. Both Mr Torstensen and Ms Israelsson worked exclusively on intermittent catheters. Mr Torstensen thought that there may have been manufacturers who made both types of catheter, but he was not sure. I will assume that they were typical and that a developer of intermittent catheters in 1997 would have had no experience of developing indwelling catheters.
- Mr Torstensen accepted that the two types of catheter are regulated differently and unlike intermittent catheters, insertion of the indwelling variety is carried out by healthcare professionals since the procedure is more invasive. He maintained that nonetheless the two types of catheter do much the same job and that information about indwelling catheters could be a source of information for a skilled person working on intermittent catheters.
- Ms Israelsson said that the skilled person who was experienced in developing intermittent catheters would have been familiar with only the basic features of an indwelling catheter. She gave an example of an inflatable balloon which may be incorporated in an indwelling catheter. Ms Israelsson said that such a skilled person would not know the details of how to incorporate such a balloon.
- Although there was a difference in emphasis, the evidence from the two experts was not far apart on this issue. A person skilled in intermittent catheters would probably have had no experience in developing indwelling catheters and possibly not much experience of them at all. But he or she would know what they are and, as part of the common general knowledge, know broadly their structure, function and how they are used.
- The second and more important dispute was whether the skilled team would have been aware that there was a demand among users of intermittent catheters for an intermittent catheter with a pre-wetted hydrophilic layer.
- In its argument on inventive step Coloplast laid heavy emphasis on the fact that over a decade passed from the introduction of the LoFric product in 1983 to Coloplast's realisation, as it claimed, that such a product could be improved by allowing patients to use the catheter without the need to find an external source of clean water. That constituted a very long-felt want, Coloplast argued.
- MacGregor's response was that there was nothing new or inventive in September 1997 about the mere idea of a pre-wetted catheter. Patients had asked for it and the skilled team would have had that goal well in mind. The difficulty was that there were technical barriers in the way of achieving this result. Coloplast may have been the first to overcome such barriers leading to the launch of Coloplast's SpeediCath. Coloplast may or may not be entitled to patent production for a solution to the practical problems and indeed Coloplast has been granted such protection. But the basic idea was still obvious.
- I will develop these contentions in more detail below. I mention them now to emphasise the importance of a key part of MacGregor's case: the skilled team knew well before September 1997, as part of its common general knowledge, that it would be a good idea to make a pre-wetted catheter if such a thing could be developed into a marketable product.
- Ms Cahill said that there were three main problems with the existing hydrophilic intermittent catheters in September 1997. First, adding water was fiddly, especially for patients with limited manual dexterity. Second, some patients disliked waiting for 30 seconds for the coating to become fully hydrated. Third, filling catheter packages with water led to spending a long time in a toilet cubicle and this led to embarrassment. Ms Cahill went on to say in her expert report that in the years leading up to September 1997 she and her colleagues had repeatedly told the catheter manufacturers that patients wanted a catheter package with the right amount of water contained in the package or a catheter that required no preparation and which could be used as soon as the package was opened.
- Mr Torstensen confirmed that user demand for a catheter packaged with water had reached Coloplast. He said that he had heard from users that they would like a catheter that contained water in the package or alternatively a catheter which was already wetted. It was put to him in cross-examination that he had provided no documents from catheter manufacturers confirming that a request along those lines had been made. That was true, but Mr Torstensen had been employed by Coloplast. He did not have had access to Coloplast's records and there was no reason to believe that he had access to the records of other manufacturers either.
- Ms Cahill said that among the manufacturers she approached before September 1997 with her request for a catheter package containing water was the market leader, Astra. Yet Ms Israelsson was clear that despite brainstorming sessions at Astra about how to provide a better hydrophilic catheter, this was not considered. I have no reason to doubt what either Ms Cahill or Ms Israelsson said about this. I reconcile their evidence by concluding that Ms Cahill and her colleagues did tell Astra, among other manufacturers, that patients would welcome a catheter package containing water but that at the brainstorming sessions referred to by Ms Israelsson it was not thought to be an idea worth following up. Astra was a commercial organisation and there may have been reasons why such an idea was not perceived by that company as having commercial potential.
- It does not follow automatically from the evidence of Mr Torstensen and Ms Cahill that the idea of an intermittent catheter stored in water, or in a package otherwise containing water, had attained the status of common general knowledge among developers by September 1997. Documents recording their knowledge of such an idea were lacking.
- There was disclosure from Coloplast. The only relevant document in this regard was an analysis dated February 1995 of focus groups consisting of users of the existing hydrophilic intermittent catheters and how they might be improved. In the executive summary there is a reference to:
"A 'ready for use' catheter. It is recommended to make a catheter with the same low friction as the existing one, but make it ready for use as soon as the catheter bag is opened."
- This does not necessarily refer to the water being contained in the catheter package. In fact, under two lists of 'Suggestions for Improvements', taken from two separate meetings, the document refers to 'Product without water' and 'Catheter without water'. These further lists suggested that the executive summary was referring to a dry catheter that would not require wetting before use.
- However, there was evidence filed in other litigation which was made available in this trial. In about 2005 Coloplast brought proceedings in the Patents County Court against Hollister Limited for infringement of a different patent, European Patent (UK) 0 923 398 ("the PCC Action"). I was told that the action settled. Coloplast's evidence in the PCC Action included an expert report of Mogens Svanum dated 15 September 2005, which was exhibited to the expert report of Mr Torstensen in these proceedings. In the mid-1990s Mr Svanum was the Manager of the Development Department for the Continence Care Division at Coloplast. He was in charge of the team that developed what became the SpeediCath. Mr Torstensen was part of the team.
- I have mentioned Astra's LoFric product, first sold in 1983. For many years it was the only hydrophilic coated urinary catheter on the market. Ten years later Coloplast launched a similar product called Conveen EasiCath, generally referred to in the evidence as the EasiCath. Mr Torstensen described this as being essentially a copy of the LoFric catheter. As with the LoFric product, the catheter was stored dry. The user opened the packaging, added water, waited for 30 seconds during with the hydrophilic coating was activated and then used the catheter.
- In his report Mr Svanum discussed information received by his team at Coloplast regarding the EasiCath as part of the development of the SpeediCath product (essentially the product of the Patent):
"31. Feedback continued to be received from the medical profession, our clinical advisory boards, focus groups, and end users and the results collated over a period of time. What emerged from these results was that whilst the EasiCath was generally well received and accepted, there were a number of practical issues that were significant concerns to end users. One of the main concerns was the need for water to activate the hydrophilic coating of the catheter. Water can be messy but a greater difficulty arises when the end user is away from home or travelling. In such circumstances the end user had not only to rely upon facilities with water being available, but also facilities that are accessible and reasonably hygienic. Importantly, even if water was available it needed to be of a satisfactory quality to avoid risk of infection.
…
33. Further, many end users also have poor manual dexterity and filling the catheter bag through a narrow opening proved difficult. Those difficulties were made worse when outside of the normal environment, for example trying to get the open end of a long package under a tap to fill it. There were also difficulties with connecting the catheter to a collecting bag when no toilet was available. Users reported that they wanted an easier system, a set or kit that would have the necessary water with it, but that was also hygienic and maintained sterility.
…
39. In June of 1996 whilst work was progressing on the New Conveen EasiCath product we received a request from Coloplast's Italian and German subsidiaries that we develop and produce a kit or set type product which would have water with it to activate the catheter coating, so that the user would no longer be dependent upon locating facilities or be concerned with their adequacy. This would be of particular benefit amongst other things when travelling, allowing the user a much greater degree of freedom.
40. We considered this request and realised that a product of this type was potentially viable as there was already some demand."
- It is apparent that by around June 1996 Coloplast had received feedback from the medical profession, clinical advisory boards, focus groups and end users. The results from this feedback were collated over a period of time. They indicated a demand for a kit which would have water with it. The documents recording these results were not disclosed by Coloplast in the present litigation.
- The executive summary dated February 1995, which was disclosed, reflects only results from three focus groups organised in Denmark and Italy. There is no mention of results from the medical profession, clinical advisory boards, end users other than through focus groups or indeed other focus groups. The executive summary does not convey the key point identified by Mr Svanum, namely that in around 1995 and 1996 the main concern among end users was that they wanted a catheter kit with water and that this was known to Coloplast.
- Mr Svanum's evidence in the PCC action is entirely consistent with what Mr Torstensen said in the present action: it was common general knowledge among developers of intermittent catheters in September 1997 that there was a market demand for a catheter packaged with water so that the coating of the catheter was ready to insert. I accept that evidence.
Disclosure in the IPEC
- Following the trial, for reasons that will become apparent, I was provided with a transcript of the case management conference in this action. At that hearing MacGregor applied for disclosure of 9 categories of documents held by Coloplast. Category 1 consisted of the documents disclosed in the PCC Action. Categories 2 to 9 were categories of documents mentioned by Mr Svanum in his evidence in the PCC Action plus certain other documents. Mr Austen, for MacGregor, told me that the documents disclosed in the PCC Action were all contained in two or three boxes held by Bird & Bird, the solicitors who had then acted for Coloplast, and that consequently there would be little cost to Coloplast in providing them.
- As I have mentioned, the PCC Action concerned a different patent. Also, the rules on disclosure in the PCC at the time of the PCC Action were not the restricted disclosure rules which now apply in the IPEC. It was to be expected that the disclosure in PCC Action would have been more extensive than that required in an equivalent IPEC action.
- In response to MacGregor's application Dr Baran told me that the documents held by Bird & Bird were in Danish and would have to be translated for Coloplast's present lawyers to review them and that this would cost £50,000. As for finding and reviewing categories 2 to 9, this would cost a six figure sum. An issue of potential patient confidentiality was also raised. Dr Baran submitted that the disclosure sought by MacGregor sounded like a task greater than preparing for the entirety of the rest of the trial.
- Having heard quite extensive argument, I ordered disclosure of categories 2-9 sought by MacGregor but only in so far as they were contained in category 1, i.e. to the extent that they were held by Bird & Bird as documents disclosed or disclosable in the PCC Action. That disclosure was subsequently provided by Coloplast.
- By the time of the trial I no longer recalled the dispute over the scope of Coloplast's disclosure. In relation to a key issue of fact – whether Coloplast had been told that there was a demand for water-stored catheters – I was shown the analysis of February 1995, the only disclosed Coloplast document relevant to this issue. It was not until I came to write the judgment that I noticed that Mr Svanum's evidence in the PCC Action had established that there must be other documents held by Coloplast which were highly relevant to this issue. In fact the analysis of February 1995 gave an impression quite different to that of Mr Svanum's evidence in the PCC Action and the apparent effect of these undisclosed documents. After I had handed down a draft of this judgment, I raised the matter with counsel. They made some preliminary comments and I was sent a copy of the transcript of the case management conference.
- I was aware of the risk that I might draw a false inference from the difference between what Mr Svanum had said and the impression given by Coloplast's disclosure. In a further email sent via my clerk I asked the parties to attend a short hearing. I explained that I wished to better understand what had happened.
- A few days later I was sent a witness statement made by Paul Inman dated 17 October 2018. Mr Inman is a partner at Gowling WLG (UK) LLP, responsible for the conduct of this litigation for Coloplast. Mr Inman's statement ran through the requests for disclosure from MacGregor, the exchanges at the case management conference and my order. He said that in March 2018 MacGregor had written to Coloplast seeking the disclosure of further documents but that all the documents requested had already been disclosed.
- This was followed by witness statement dated 19 October 2018 from Sara Ashby, the partner at Wiggin LLP responsible for the conduct of the action on behalf of MacGregor. According to Ms Ashby Coloplast had initially suggested that there should be no disclosure in these proceedings and thereafter when MacGregor repeatedly pressed for disclosure Coloplast had at each stage been highly resistant. In McGregor's view Coloplast even failed to disclose all the documents which I ordered to be disclosed at the CMC. Apparently only 8 documents were disclosed.
- Neither party sought a further hearing. Having read these two witness statements I saw no value in one. I am not in a position to resolve whether Coloplast complied with the order for disclosure made at the CMC and it would not be in the interests of procedural economy to pursue the matter. I therefore assume that Coloplast was in full compliance.
- However, Mr Inman's statement fails to address the issue I raised. By the time of the trial, and probably well before that, it was clear to the parties that the key issue of fact to be resolved at the trial was whether Coloplast and other manufacturers of catheters knew in September 1997 that users of intermittent catheters wanted a wet-stored catheter. If so, it was likely that by inference the skilled team would have been aware of the idea of a wet-stored catheter at that date as part of their common general knowledge. In opening Dr Baran went so far as to submit that the three cited items of prior art did not advance MacGregor's case on the common general knowledge (Day 1, p.12). In other words, Coloplast had taken a very clear view that MacGregor's case was going to stand or fall on whether the idea of a wet-stored catheter was known, irrespective of argument over the cited prior art.
- In the draft of this judgment provided to the parties before Mr Inman's statement, I indicated that there was a suspicion that Coloplast had not given disclosure of documents identified by Mr Svanum even though and possibly because Coloplast believed that the documents would have corrected the impression given by the executive summary of February 1995. Mr Svanum's report strongly implies that those documents would have revealed that by September 1997 Coloplast knew that users wanted a wet-stored catheter. Mr Inman did not state otherwise and has not lifted the suspicion. His statement suggests rather that given the way the case management conference went, Coloplast was advised that highly relevant documents need not be disclosed. I make two observations.
- First, it is not satisfactory that an order for specific disclosure, however framed, should result in a party concluding that it need not disclose documents which to its knowledge relate to a material and central issue and which it knows to be in its possession.
- From 1 January 2019 the disclosure pilot for most of the Business and Property Courts will enter into force (see CPR 100th Update, Annex B). All five proposed disclosure models A to E will require disclosure of known adverse documents (as there defined). The pilot will not apply in the IPEC. Nonetheless, hereafter where disclosure is ordered at all in the IPEC it is likely that disclosure will include, as a basic minimum, known adverse documents. Since such documents do not require a search, save in unusual circumstances their disclosure will accord with the IPEC goal of minimising costs.
- My second observation is that although the IPEC procedure is designed to keep disclosure to the essential minimum – generally, running an increased risk of excluding relevant documents from disclosure is a price well worth paying for the savings in cost and time – this should not be allowed to result in a real likelihood that the court will reach a false understanding of an important issue. The overriding objective, including making sure that the case is heard fairly, must always take precedence. Notwithstanding the IPEC rules, an application for disclosure should be pursued, even after the CMC where appropriate, if it is based on solid evidence, i.e. more than speculation however keenly felt, that documents likely to shed light on an important issue are held by the opposing party. However, such applications must be as narrowly focussed as is reasonably possible. Part of the difficulty over disclosure in the present case may have been a lack of focus by MacGregor.
Construction
The law
- The law on construction and the scope of claims set out by the Supreme Court in Actavis UK Ltd v Eli Lilly Co [2017] UKSC 48; [2017] RPC 21 was recently considered and further explained by the Court of Appeal in Icescape Ltd v Ice-World International BV [2018] EWCA Civ 2219.
- Icescape was handed down after the trial but that creates no difficulty because Coloplast did not run a case on equivalents. Counsel on both sides were content to argue construction on the basis that I should construe the claims according to the principles which applied before Actavis. I am not sure that the first stage referred to by Lord Neuberger (see Actavis at [54]), as explained in Icescape, is an approach exactly the same as construction of a claim according to the principles set out in Kirin-Amgen v Hoechst Marion Roussel Ltd [2005] RPC 9. In any event, I will construe each claim according to what the person skilled in the art, reading the claim in the context of the specification as a whole, would have understood the patentee to be using the language of the claim to mean.
The parties' statements of case on construction
- The main issue between the parties on construction concerned two potential embodiments of the invention claimed in the Patent. In the first, the water is introduced into contact with the catheter during manufacture of the assembly. The hydrophilic catheter is thus stored in a wet and activated form. I will call this the 'ready-wetted' configuration.
- In the second embodiment, the catheter is dry until activated by the user squeezing the sponge. There could be means of storing the water other than in a sponge, but counsel spoke mostly of the sponge means. The water thus activates the hydrophilic layer just before the catheter is used. I will call this the 'user-activated' configuration. I will refer to both collectively as 'water-stored catheters'.
- At the CMC the parties agreed to exchange statements of case on construction, which proved to be helpful.
- MacGregor's statement said:
"The liquid swelling medium is not in contact with the catheter immediately following manufacture of the assembly, but is put into contact with the catheter by the eventual user of the catheter assembly. In the context of claim 1 of the Patent, 'prior to use' therefore means 'immediately prior to use'.
- It also said:
"Claim 1 does not require that the liquid swelling medium is accommodated in a compartment separate from that of the catheter. It may be stored within the cavity of the catheter package in any manner separate from the catheter itself."
- In other words, claim 1 is confined to the user-activated configuration.
- Coloplast's statement on construction said:
"Within the meaning of the terms used in the claim, the liquid swelling medium and the catheter are introduced into the cavity of the catheter package made of gas impermeable material by the manufacturer during manufacture. The liquid swelling medium may not be in a closed and separate compartment having walls of a gas impermeable material but may be in a storage element or separate compartment which is liquid or vapour permeable."
- Although this was not as clear as it might have been, Dr Baran stated that Coloplast's construction of claim 1 encompassed both the ready-wetted and user-activated configurations. In the latter case, although the water would be stored separately there could be no liquid or vapour impermeable barrier between the water and the catheter.
The description of the invention in the Patent
- I begin with paragraphs [0011] and [0013]:
"[0011] In a first series of embodiments of the urinary catheter assembly of the invention the catheter package as a whole is made of a gas impermeable material and the compartment for the liquid swelling medium is in liquid flow communication with the cavity for accommodation with the catheter."
…
"[0013] The compartment for the liquid swelling medium is entirely integrated with the cavity for the catheter, whereby the hydrophilic surface layer of the catheter will be activated immediately after completion of the production process, when the swelling medium has been introduced into the package. The gas-impermeable walls of the package will then product the activated coating from drying out and provide a long time preservation of the low friction surface characteristic of the catheter until the moment of actual use."
- Whatever other embodiments there may be, these paragraphs leave no doubt (subject to the wording of the claims) that the invention is intended to encompass the ready-wetted configuration.
- The specification continues with a more detailed description of this 'first series of embodiments'. Paragraph [0017] states:
"[0017] In the following, the invention will be explained in more detail by means of various embodiments illustrated in the accompanying drawings …"
There follow references to figures 1 to 3. There is also reference to figures 4 to 6 which, a little confusingly, are said not to form part of the invention, and to figures 12 and 13. There are no other figures in the Patent as granted. The skilled reader would assume that there had been substantial editing of the specification during prosecution, which may be an explanation for an occasional lack of clarity.
- Paragraph [0018] states:
"[0018] In the embodiments shown in figs. 1 and 2 the urinary catheter assembly of the invention is intended for intermittent catheterisation of the bladder of a user and comprises a urinary catheter 1 having a catheter tube 2 with cross-sectional and longitudinal dimensions suitable for introduction of the catheter through the urethra."
- Figures 1 and 2 look like this:
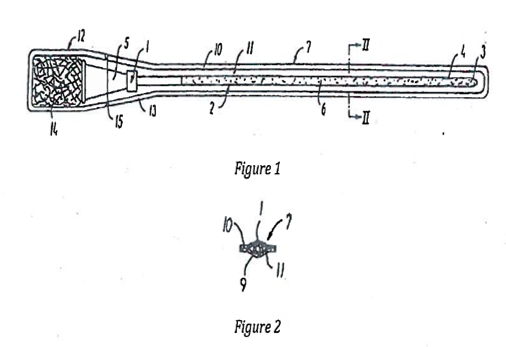
- Paragraph [0023] is part of the commentary on figures 1 and 2:
"[0023] In order to maintain the swelling medium in liquid state until the actual preparation of the catheter the liquid swelling medium is confined in the embodiment shown in a storage body 14 which as described above may be of a spongy or gel-like material located in the compartment or end section 12."
- Mr Mitcheson submitted that the reader would assume that paragraph [0018] was moving on from the 'first series of embodiments' of paragraph [0011] to a new set of embodiments. I agree. Figure 1 as explained, particularly bearing in mind the word 'confined' in paragraph [0023], does not seem to illustrate the ready-wetted configuration.
- But I do not see that this supports MacGregor's construction. The specification continues by making it clear that the ready-wetted configuration as well as the user-activated configuration is intended to fall within the scope of the invention claimed:
"[0029] Due to the gas-impermeability of the package 7 it is not necessary to use a body 14 of spongy material to accommodate the liquid swelling medium. The swelling medium may be introduced in the package during the assembling operation prior to completion of the welding and will thereby immediately prepare the hydrophilic coating. The package will itself prevent the coating from drying out and preserve the low friction character of the surface coating to keep the catheter in a ready to use condition at all times. This would have the inherent advantage that no preparation step is required immediately prior to use, whereby the operation will be reduced to opening of the package 7 for immediate withdrawal of the catheter without the delay resulting from the required preparation period.
[0030] Since the preparation period is very short and the possible presence of surplus swelling liquid in the package may be uncomfortable to the user who in many situations will have to carry one or more catheter assemblies with him or her, it would frequently be preferred, however, to keep the swelling medium confined in the spongy body 14 until the moment of actual use."
- I therefore take the view that the description indicates to the reader that the invention covers both the ready-wetted and user-activated embodiments. This must be subject to the words of claim 1.
Claim 1
- In MacGregor's skeleton and statement on construction, an argument relating to claim 1 itself was raised. Integer 1.5 was relied on:
[a hydrophilic coating] intended to produce a low-friction surface character of the catheter by treatment with a liquid swelling medium prior to use of the catheter
- MacGregor's statement on construction (in a passage not quoted above) implied that the 'intention to produce' requires such an intention on the part of the user of the catheter. Therefore the claim could only cover the user-activated embodiment. I disagree. I take the view that the invention does not include a subjective element and I construe this part of the claim to mean that the coating must be suitable for producing a low-friction surface.
- MacGregor's argument continued: a natural reading of 'treatment with a liquid swelling medium prior to use of the catheter' meant immediately prior to use. It did not mean during manufacture, which could be years prior to use.
- Again, I disagree. If there is any ambiguity in the meaning of 'prior to use of the catheter' in claim 1, the body of the specification clears it up: it could be during manufacture of the assembly.
- I accept Coloplast's contention that claim 1 covers both embodiments.
- Coloplast went further, arguing that claim 1 did not cover embodiments in which the water was in a completely closed and separate compartment made of thermoplastic film containing aluminium – what Dr Baran called the 'ampoule embodiment'.
- Paragraph [0022] refers to the water being accommodated in a 'compartment', but paragraph [0021] identifies the compartment to be a widened end section of the cavity containing the catheter. The cavity is created and defined by the part-aluminium thermoplastic film surrounding it. This is confirmed by figures 1 and 2 to which paragraphs [0021] and [0022] refer.
- Figures 1 and 2 do not show any barrier separating the compartment accommodating the water from the cavity storing the catheter. Paragraph [0025] indicates that when the spongy body is squeezed, the water is allowed to flow freely into the cavity.
- Claim 1 expressly states that the same cavity accommodates both the catheter and the water. If the water were contained in an ampoule made of gas impermeable material, on a straightforward reading of claim 1 the water would not be in the same cavity as the catheter. I therefore agree with the further part of Coloplast's construction: that the water may not be contained in a part-aluminium thermoplastic film. It follows that the user-activated configuration of the invention does not encompass the ampoule embodiment. Nor, therefore, is the ampoule embodiment included within the term 'water-stored catheter' as I have defined it above.
- There was one further issue of construction, relevant to MacGregor's case on insufficiency. The claims do not expressly state that the catheter assembly must be suitable for storing the catheter for any stated period of time. However, the gas impermeable material used is defined in the specification:
"[0010] The term 'gas impermeable' material should be understood in this context to mean any material that will be sufficiently tight against diffusion by evaporation of the actual liquid swelling medium for a period exceeding the recommended shelf life time of the catheter assembly which could be up to five years, typically 36 months."
- In my view, the skilled team would construe the claims to cover assemblies suitable for accommodating a catheter for up to five years.
Infringement
- MacGregor accepted that if the claims encompassed the ready-wetted configuration, sales of its products infringed.
Novelty
The law
- The parties were agreed as to the law. It is sufficient for me to set out a test often referred to, taken from the judgment of the Court in General Tire & Rubber Co v Firestone Tyre & Rubber Co Ltd [1971] FSR 417 (CA), at 444:
"To anticipate the patentee's claim the prior publication must contain clear and unmistakable directions to do what the patentee claims to have invented: Flour Oxidizing Co. Ltd. v. Carr & Co. Ltd. (1908) 25 RPC 428 at 457, line 34, approved in B.T.H. Co. Ltd. v. Metropolitan Vickers Electrical Co. Ltd. (1928) 45 RPC 1 at 24, line 1. A signpost, however clear, upon the road to the patentee's invention will not suffice. The prior inventor must be clearly shown to have planted his flag at the precise destination before the patentees."
Rødsten
- Rødsten disclosed an applicator used to insert a catheter with a friction-reducing surface coating into a body canal, in particular the urethra. The user separates the foil walls of the packaging and inserts the catheter without the need to touch it. Coloplast argued that integers 1.7 and 1.9 of claim 1 were missing from Rødsten.
- Rødsten is principally concerned with a method whereby the user opens the package, adds water to activate the friction-reducing surface coating and after removing surplus water, the catheter is activated for use. It was not contended by MacGregor that there is an express disclosure of either of the Patent's two embodiments of water-stored catheters.
- Mr Torstensen said in his expert report that the following passage on page 8 by implication disclosed the ready-wetted embodiment:
"The method and the applicator according to the invention is not restricted to use in connection with a catheter of the kind which requires moistening prior to the application, but may to the same extent be used with a catheter with is packed in a ready-for-insertion state."
- However, as Mr Torstensen conceded in his report, this would bring to the skilled person's mind a gel-lubricated catheter that does not require moistening prior to use, i.e. a catheter of the type referred to in the Patent as part of the prior art. He went on to say that the same passage could also tell the skilled person that the Rødsten catheter is ready for use because it had been stored in water. He maintained this possibility in cross examination.
- Ms Israelsson was also cross-examined on the same passage, but on the basis, which she was asked by Mr Mitcheson to assume, that the skilled team reading Rødsten had been asked by users for a catheter kept in water and that this was part of their common general knowledge. Ms Israelsson accepted that on this assumption the passage quoted could only be referring to the possibility of a catheter packed in contact with liquid.
- Even on the assumption put to Ms Israelsson, I am not able to accept her answer at face value. The passage quoted allows for another possibility – as Mr Torstensen conceded, namely a catheter that does not require wetting. The passage is ambiguous and so does not provide clear and unmistakable directions to a water-stored catheter of either type.
- Turning to integer 1.9, Mr Torstensen said that a reference in Rødsten to the use of "other suitable foil materials in the form of single layer foils or laminates of plastic, metallic foils and/or paper" would necessarily direct the skilled team's mind to "gas impermeable material formed by a multiple layer thermoplastic film material comprising aluminium" of claim 1.
- Ms Israelsson said it would not. In cross-examination she accepted that if the skilled team had the idea of a water-stored catheter, a gas impermeable material would be used for the packaging. Earlier, during cross-examination in relation the Israelsson prior art, Ms Israelsson had also accepted that it was known to use thermoplastic aluminium foil as an appropriate material for packaging a catheter. That may be so, but I am not satisfied that Rødsten would necessarily lead the skilled team to using the relevant type of packaging.
- Claim 1 does not lack novelty over Rødsten.
Israelsson
- Israelsson is a PCT application which discloses a hydrophilic urinary catheter with a water container, shown in the figures as a sachet. The water container is positioned within a urine collecting bag. The user can release the water into a receiving area to wet the hydrophilic coating of the catheter. The specification describes this as providing an 'on tap' supply of lubricant for the catheter.
- Coloplast's pleaded case was that Israelsson disclosed none of elements 1.6 to 1.9 of claim 1. In summary, the Israelsson invention had no package, whether made of a multiple-layer thermoplastic film material comprising aluminium or otherwise, which contained both the catheter and the water in the same cavity.
- Ms Israelsson gave evidence about her own PCT application. She had asserted in her report that it did not disclose elements 1.6 to 1.9. She was cross-examined on the basis that the main distinction between Israelsson and the Patent which she had identified was the fact that Israelsson requires a 30 second holding time between releasing the water to wet the catheter and use. Although Ms Israelsson agreed, given what she said in her report I am not sure that is right. In any event, I did not understand her to alter her earlier stated view that integers 1.6 to 1.9 of claim are absent from Israelsson.
- Mr Torstensen argued in his report that although the water and the catheter were stored in separate compartments, both compartments were within the same receptacle. He implied that if on a correct construction claim 1 includes an embodiment in which the catheter is not stored in contact with the water (such as the sponge embodiment), as I have found, this was the same thing.
- In cross-examination Mr Torstensen conceded that it was not the same thing. I believe he was right to do so. In the Patent, the water and the catheter may optionally be stored separately, but they are stored within the same cavity. On the construction of claim 1 which I have accepted, this means that there must be no gas impermeable barrier between the water and the catheter. In Israelsson the wetting fluid container is said (at page 5, lines 17-21) to be preferably made of material impermeable or substantially impermeable to ethylene oxide and the fluid. There is no disclosure of storage of the fluid using a container made of a gas permeable material.
- It was suggested by MacGregor that one alternative disclosed in Israelsson constituted an anticipation of claim 1. The disclosure came in a passage at the end of the description, immediately before the claims:
"In the exemplary embodiments hereinabove described with reference to the Figures of drawings the supply of wetting fluid for wetting of the hydrophilic urinary catheter takes the form of a separate sachet integrated into the wetting receptacle. It will be appreciated by those versed in the art that the supply could also take the form of an integrally formed chamber in the material of the wetting receptacle without departing from the spirit if the invention."
- It was put to Ms Israelsson that the alternative embodiment referred to in the second sentence was the same as that disclosed in figure 1 of the Patent. Ms Israelsson seemed unclear as to what form the alternative embodiment was intended to take. It was apparently not an embodiment with which she was familiar.
- The best I can make of that sentence is a suggestion that the sachet containing the water could be located within the material from which the wetting receptacle – containing the catheter – is made. This is as opposed to the sachet being located in the wetting receptacle, such as being fixed to the inner surface of the wetting receptacle, see page 5, lines 25-27. Given the material from which the sachet is said to be preferably made, neither arrangement would anticipate claim 1.
- Claim 1 does not lack novelty over Israelsson.
Conclusion on novelty
- Claim 1 is not anticipated by either Rødsten or Israelsson. Consequently neither are claims 3 and 5.
Inventive step
Rødsten
Primary evidence
- The features of claim 1 of the Patent missing from Rødsten were the ideas of a water-stored catheter and a package made of a thermoplastic film comprising aluminium.
- I have found that the notion of a ready-wetted catheter was part of the common general knowledge. Ms Israelsson said that if this were the case, the disclosure in Rødsten would lead the reader to think that it was referring to a ready-wetted catheter. Mr Torstensen stated that the reader would believe this even without assuming such common general knowledge. I was not able to accept that evidence for reasons I have explained, but what Mr Torstensen said is consistent with Ms Israelsson's position once such common general knowledge is assumed.
- Both experts indicated that if the skilled team had the idea of a ready-wetted catheter, thermoplastic film comprising aluminium was a known and obvious material to use for packaging the catheter.
- On the primary evidence alone claim 1 is obvious over Rødsten.
Secondary evidence
- Coloplast laid great emphasis on long felt want and commercial success. Taking first the question of long-felt want, it is important that the want must be for the invention as claimed, the invention having been made possible by the inventive concept. In the present case, as Dr Baran asserted, the alleged inventive concept was the idea (no more) of a water-stored catheter, one embodiment of which was a ready-wetted catheter. He then added that the inventive concept also included the idea of thermoplastic film comprising aluminium to be used for packaging the catheter. The latter adds nothing since such material was shown on the evidence to be a known and obvious material to use if, as would be the case, it was necessary to prevent evaporation of the water.
- I have found that the key idea of a ready-wetted catheter was part of the common general knowledge. There can have been no long-felt want for an invention made possible by only that concept.
- The lack of a water-stored catheter on the market until the development of the SpeediCath could be explained by reasons other than a water-stored catheter being outside the obvious contemplation of the skilled team. The most likely candidate in the present case was the inability on the part of the manufacturers to come up with a commercially viable product until Coloplast managed it. But the fact that the inventive concept was known at the priority date is by itself fatal to Coloplast's argument of long-felt want.
- In the absence of a long-felt want, Coloplast's evidence of commercial success can add nothing to its case on inventive step.
- Claim 1 lacks inventive step over Rødsten.
Israelsson
- The difference between Israelsson and the invention of the Patent is that although Israelsson discloses a kit with water, it discloses neither a ready-wetted catheter nor a user-activated catheter, the latter defined above to exclude the ampoule embodiment. In Israelsson water is stored with the catheter but in a separate sachet.
- Mr Torstensen's evidence was that if the skilled team had the idea of a ready-wetted catheter, the invention of claim 1 would be obvious over Israelsson. It would be obvious to store the water with the catheter rather than in a separate sachet. I did not understand Mr Baran to challenge this. The main distinction relied on by Ms Israelsson, namely that the invention of the Patent required the catheter and the water to be stored in the same cavity, ceases to be a meaningful distinction if the idea of a ready-wetted catheter is part of the common general knowledge.
- Thus, according to both experts it was obvious to package a catheter containing sterile water with gas impermeable packaging such as a thermoplastic film containing aluminium.
- Claim 1 lacks inventive step over Israelsson.
Shibatani
- Shibatani is a Japanese patent which discloses a drug-delivery device using a catheter. The catheter is an indwelling catheter with a hydrophilic layer. The catheter is stored in contact with the drug in a sterile casing. Two embodiments are described. In both it is stated that the hydrophilic resin coating layer of the catheter is held in the drug for some days and so that the catheter could be used immediately to administer the drug. The specification states that the catheter has shown best results when applied as a urinary catheter.
- Coloplast's primary position was that Shibatani would have been of no interest to the skilled team because it concerned an indwelling catheter used for a purpose quite different from that of an intermittent urinary catheter. I do not accept that. I have found that claim 1 covers an indwelling urinary catheter. Therefore the skilled team would include a developer interested in improvements to indwelling catheters. Even if the skilled team did not contain such a developer and were interested only in intermittent catheters, to the extent that the structure and function of the two types of catheter overlap, new information about indwelling catheters in the area of overlap would have been of interest to a skilled developer of intermittent catheters. It would have been perverse to ignore such information. This would include a means of providing a low-friction catheter with the minimum of fuss from the user's point of view.
- Given my finding that that the idea of a ready-wetted catheter was part of the common general knowledge and that it was common ground that the LoFric and EasiCath products were also part of the common general knowledge, the argument on inventive step in respect of Shibatani becomes somewhat artificial. However, with that common general knowledge, claim 1 is also obvious over Shibatani.
Claims 3 and 5
- Little was said about claims 3 and 5. It is enough for me to consider them in relation to Rødsten. The further features of claim 3 over claim 1 were that (a) the catheter as a whole is made of a gas impermeable material and (b) the package is formed from two sheets of the material connected by a gas impermeable joint. Mr Torstensen said that Rødsten discloses the first of these and that the second is inevitable if aluminium-plastic laminates are used to make the package. He was not challenged on this. Ms Israelsson said nothing in her report about claim 3 being inventive over claim 1.
- Coloplast did not allege that there was anything separately inventive in claim 5
- Claims 3 and 5 lack inventive step over Rødsten.
Insufficiency
- As I have said, MacGregor's answer to Coloplast's case on long-felt want was that the idea of a water-stored catheter was known, yet implementing the idea to make a marketable product was not possible until technical difficulties had been resolved. This feeds into MacGregor's argument on insufficiency. MacGregor argued that the Patent explained the known idea of a water-stored catheter but did not inform the reader how to make such a thing – how to overcome the technical difficulties.
- During the trial a variation on the theme began to emerge, a case on what is sometimes called Biogen insufficiency, i.e. an alleged failure to disclose the invention sufficiently across the breath of the claim. It was not pursued by Mr Mitcheson in closing. If ever there was an intention to plead Biogen insufficiency, in my view that allegation did not emerge with adequate clarity in the Amended Grounds of Invalidity.
The law
- It was common ground between Mr Mitcheson and Dr Baran that s.72(1)(c) of the Patents Act 1977 does not require that the specification enables the skilled person to make a commercially marketable embodiment of the invention. In Mentor Corp v Hollister Inc [1993] RPC 7, the Court of Appeal discussed insufficiency on the assumption that producing a workable prototype would be enough (at p.17). Whether in the form of a prototype or otherwise, if the Patent enables the skilled person, using his common general knowledge and without undue burden, to make or otherwise obtain a product as claimed or carry out a process as claimed, the subsection is satisfied (see Eli Lilly & Co v Human Genome Sciences Inc [2008] EWHC 1903 (Pat) at [239], the ruling on insufficiency approved by the Supreme Court at [2011] UKSC 51; [2012] RPC 6, at [132]-[139].) When discussing undue burden in Eli Lilly, the Supreme Court approved the frequently used distinction between a research programme and routine trials (at [132]).
The evidence
- Mr Torstensen said in his report that if a skilled person had tried to make a ready-wetted catheter at the filing date he or she would have discovered that it could not be done with the hydrophilic coatings known at the time. Mr Torstensen referred to experiments done by Coloplast mimicking the effect of leaving the catheter in water for one to two years. I have found that the claims require that the assembly be suitable for storing the catheter for up to five years, but there was no reason to believe that this made any difference.
- The problems that emerged from these experiments were an unpleasant smell, a change of catheter colour, loss of flexibility in the PVC catheter and the hydrophilic coating becoming detached from the catheter. Mr Torstensen said that it was apparent to the team that a catheter packaged in water would require complex and lengthy research work and that such work was beyond the capability of the team. According to Mr Torstensen the known hydrophilic coatings were not suitable for storing the catheter in contact with water. He also said that the gas impermeable packaging would require the use of electron beams as a means of sterilisation and that this tended to cause the hydrophilic layer to lose its low friction character.
- A separate team at Coloplast, specialising in materials technology, took over the project. The goal of this team was to identify a catheter material other than PVC and a suitable hydrophilic coating.
- Ms Israelsson conceded in cross-examination that the skilled team, having read the Patent, would have to embark on a research project in order to make a catheter assembly as claimed. That sounded like Coloplast's own expert finishing off its case on insufficiency, but although a witness may agree with counsel's use of magic terms such as 'research project' or 'research programme', it is still necessary to know what the witness means by such terms.
- Mr Torstensen accepted in cross-examination that the problems of smell and colour would not prevent the skilled team from making a product, even though it would not be a marketable product. Thus, if the research project had involved only solving these two problems the Patent would not lack sufficiency.
- The difficulties of finding a suitable material for the catheter and a suitable hydrophilic coating fell into in a different category. This was because it was necessary to solve those two problems into order to make a urinary catheter assembly falling within claim 1 as I have construed it.
- Mr Svanum discussed the work facing Coloplast at the time in his evidence in the PCC Action:
"38. The hydrophilic coating we then used for the EasiCath was not suitable as it could not be kept in a wetted condition for long and certainly not for the proposed shelf life of the new product. A new hydrophilic coating would be needed which would be a major development and which would take some time. A new catheter would also be required as PVC swells when immersed in water for any length of time. The product would need a shelf life of 3 years. As an initial step Coloplast's Research Centre had been instructed to start research and to begin work on developing a new hydrophilic coating."
- Mr Svanum's report was apparently made available in proceedings before the EPO Technical Board of Appeal concerning the Patent. Coloplast filed a corrective statement from Mr Svanum dated 21 April 2009 in the TBA proceedings, explaining what he had meant in his earlier paragraph 38. He said that finding a suitable hydrophilic coating was only regarded as a 'major development' because Coloplast had no specialist knowledge of its own in the field of hydrophilic coatings. He added that that for environmental reasons and because Coloplast's supplier of uncoated PVC catheters was developing a rival product, Coloplast decided to find a non-PVC alternative. He suggested that nonetheless the existing EasiCath PVC catheter could possibly have been used to make a catheter assembly as claimed.
- In the end I must primarily rely on the evidence from the witnesses in these proceedings. As I have indicated, Mr Torstensen in his report stated that the two problems of finding a suitable catheter material and a suitable hydrophilic coating would have prevented the skilled team from making a catheter package as claimed without a significant amount of research and development.
- In cross-examination he accepted that there were ways forward which the skilled team could have adopted. But this did not establish that the work to be done by the skilled team would be routine, particularly with regard to the coating.
- Mr Torstensen was taken to one of the 13 patents and one design referred to in paragraph [0002] of the Patent, namely the first of them: EP A 0 217 771 ("the 771 Patent). This was an Astra patent claiming a method of applying a hydrophilic coating, but not concerned with coatings for water-stored catheters. Mr Torstensen accepted that of the many coatings disclosed by this document, the preferred coating was polyvinylpyrrolidone (PVP). The significance was that Ms Israelsson had said that in the course of the Astra trials, during which there was simulated storage of the catheter in water for 1-2 years, a catheter dipped in PVP had proved satisfactory. Dr Baran argued that on this evidence it must have been a routine matter to find a suitable coating for a catheter that would survive storage in water.
- One reason I am unable to accept this argument is that it has the character of hindsight reasoning. Paragraph [0002] merely sets the scene by identifying the sort of catheter with which the invention is concerned. It does not expressly tell the reader that a suitable hydrophilic coating is to be found among the prior art listed. Even if the skilled team were to interpret the paragraph in this way, there was no reason to focus on the preferred coating of the 771 Patent.
- Another difficulty I have is that in cross-examination Ms Israelsson said that Astra's coating was proprietary and its formula not available to others. It was not established that merely being told to use PVP would be sufficient information to enable the skilled team to make a coating that could be stored in water for up to five years. It seems that PVP worked, but it may have been a particular formula using PVP that was needed.
- The third problem is that, as I have found, Astra had been told that the market wanted a water-stored product. It was put to Ms Israelsson that the reason Astra did not meet that demand before Coloplast was that Astra could not find a coating sufficiently stable in water. Ms Israelsson answered (Day 2, p.240):
"No, I do not think so, but I cannot be sure. I do not know. It could be others."
- I think that if finding a stable coating had really been a routine matter for Astra Ms Israelsson would have unequivocally dismissed the problem of finding such a coating as a realistic reason why Astra had not marketed a water-stored product sooner than it did.
- In my view, based on the evidence of both Mr Torstensen and Ms Israelsson, the specification does not disclose the invention clearly and completely enough for it to be performed by the team skilled in the art.
Conclusion
- The Patent is invalid for both lack of inventive step and insufficiency. Had it been valid, MacGregor would have infringed the Patent.


