Neutral Citation Number: [2021] EWHC 1026 (Pat)
Case No. HP-2015-000003 and HP-2020-000014
IN THE HIGH COURT OF JUSTICE
BUSINESS AND PROPERTY COURTS OF ENGLAND AND WALES
INTELLECTUAL PROPERTY LIST (ChD)
PATENTS COURT
Rolls Building
Fetter Lane
London, EC4A 1NL
23 April 2021
Before :
MR JUSTICE MEADE
- - - - - - - - - - - - - - - - - - - - -
Between :
|
|
(1) ALCON RESEARCH LLC
(2) ALCON PHARMACEUTICALS LIMITED |
Claimants
|
|
|
- and - |
|
|
|
(1) ACTAVIS GROUP PTC EHF
(2) ACCORD-UK LIMITED
(Defendants in HP-2015-000003)
(1) PHARMATHEN SA
(2) ASPIRE PHARMA LIMITED
(Defendants in HP-2020-000014)
|
Defendants
|
Andrew Waugh QC and Anna Edwards-Stuart (instructed by Bristows LLP) for the Claimants
Justin Turner QC and Daniel Selmi (instructed by Innovate Legal) for the Defendants
Hearing dates: 16-18 and 22 March 2021
- - - - - - - - - - - - - - - - - - - - -
Approved Judgment
I direct that pursuant no official shorthand note shall be taken of this Judgment and that copies of this version as handed down may be treated as authentic.
Covid-19 Protocol: This Judgment was handed down remotely by circulation to the parties’ representatives by email and release to Bailii. The date for hand-down is deemed to be 23 April 2021.
Mr Justice Meade:
Introduction. 3
Conduct of the trial 3
The issues. 4
The witnesses. 4
The skilled team.. 6
The skilled team - applicable law.. 7
The facts, application of the law.. 10
The common general knowledge. 13
Agreed common general knowledge. 13
General pharmacological principles. 13
Prodrugs. 14
The anatomy of the eye. 14
Glaucoma. 15
Treatment for glaucoma. 15
Prostaglandins. 16
Prostaglandins and the treatment of glaucoma. 17
Animal models. 17
Prostaglandin drugs in 1993. 17
Disputed common general knowledge. 18
Prostaglandins as therapeutic agents to treat glaucoma. 19
Prostaglandins, prostanoid receptors, and their classification. 19
Fluprostenol 21
The Patent 21
Example 5. 23
Example 6. 24
Claims in issue. 24
Technical contribution of the Patent 26
An Issue of claim interpretation. 27
Validity. 27
Anticipation - the law.. 27
The allegations of anticipation in this case. 27
Obviousness over Stjernschantz. 29
Legal principles. 29
Teaching of Stjernschantz. 29
Pozzoli analysis. 33
The Defendants’ case. 34
Evidence relied on by the Defendants. 37
Conclusion on obviousness. 38
Obviousness/insufficiency squeeze. 38
Conclusions. 38
1. These actions concern European Patent (UK) No. EP 1 920 764 (the “Patent”) which protected the drug travoprost until its expiry on 3 August 2014. Thereafter, an SPC based on the Patent, SPC/GB12/038, (the “SPC”) was in force until 28 May 2017.
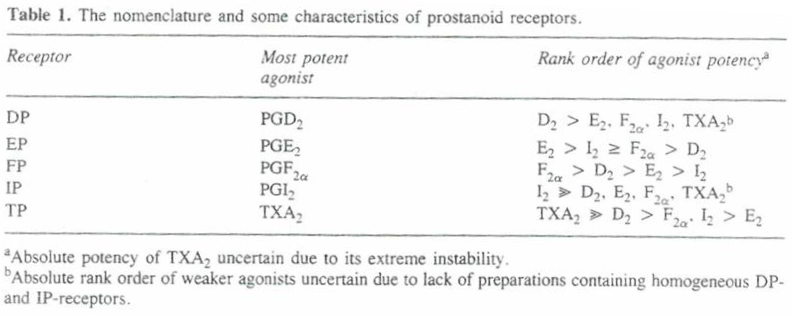
2. The Patent was opposed in the European Patent Office (“EPO”) and upheld in amended form by a decision of the TBA of 21 April 2016 (T1872/14 Travoprost/ALCON), to which I will refer below.
3. The TBA’s decision was on the basis of different prior art, different evidence, and different arguments, so in many respects it is of only limited relevance.
4. The Patent relates to the use of travoprost (fluprostenol isopropyl ester (“FIE”), a prostaglandin F2a analogue) for the treatment of glaucoma and ocular hypertension (in this judgment I will generally, for brevity, just refer to glaucoma as the condition to be treated, and not refer repeatedly to ocular hypertension; the relationship between them is explained below). Its priority date is 3 August 1993.
5. Although the Patent and the SPC have both expired, this trial has been necessary because interim injunctions were obtained on the basis of them in respect of which cross-undertakings in damages were given. If the Patent is invalid then those cross-undertakings will come into force.
6. It is unnecessary to go any further into the procedural history in this judgment, or to refer in any detail to the different Defendants and their relationships. All I need say is that the only active Defendants remaining are the second Defendant in each action. I will refer to them together as “the Defendants”.
7. There was quite a lengthy period when the proceedings were paused, and that had the result that the expert witnesses started work on their reports several years ago, so their recollection of the process of their preparation has faded.
8. Infringement (in the event that the Patent is valid) by the Defendants’ generic products is admitted. So in essence this is now a revocation action. The only attack on the SPC is that it is invalid if the Patent is invalid, so I do not need to say anything further about it.
9. Andrew Waugh QC appeared for the Claimants, to whom I will refer as “Alcon”, with Anna Edwards-Stuart, and Justin Turner QC and Daniel Selmi appeared for the Defendants.
10. The trial was conducted fully remotely, supported by a third-party IT provider retained by the parties, and worked well. Dr Krauss, Alcon’s pharmacology expert, gave evidence from the USA, and to accommodate this, and to allow him to watch the proceedings during the evidence of his opposite number, Dr Wilson, the Court sat some time-shifted hours, outside the normal Court day.
11. I thank the Court staff and the shorthand writers for their assistance with these extended hours, and the parties for providing electronic bundles.
12. The issues narrowed in the run up to trial, and during trial. The remaining issues are:
i) The nature of the skilled team.
ii) The scope of the common general knowledge.
iii) Novelty over a patent application referred to as “EP 800” (European Patent Application No. 0 603 800). This was prior art for novelty only. Novelty has to be assessed for the claims upheld by the EPO and, if those are anticipated, in respect of amended claims put forward by way of a conditional application to amend.
iv) Obviousness over a prior art publication referred to as “Stjernschantz” (“Phenyl substituted prostaglandin analogs for glaucoma” by Stjernschantz and Resul published in Drugs of the Future, 1992 Vol 17(8): 691-704 published in October 1992).
v) An obviousness/insufficiency squeeze.
13. One of the issues that fell away during trial was an allegation that the Patent was obvious over another prior art document referred to as “Karlsson” (Abstract No. 55 entitled “Receptor profile of PhXA41, a new phenyl substituted prostaglandin ester” by Maritha Karlsson et al. published in Experimental Eye Research Vol 55, Supplement 1 in or around September 1992), either alone or in combination with Stjernschantz. Karlsson was referred to in Stjernschantz and the attack over it was closely related to that over Stjernschantz. It was a pragmatic decision by the Defendants to drop it in the light of the way the evidence had gone; it was reasonable to run it up until then.
14. I will refer below to a point about the interpretation of the claims (the meaning of “for use”), but nothing turned on it and in the end I do not think there was a dispute about it.
15. Each side called two expert witnesses: a pharmacologist and a medicinal chemist. It was common ground that the skilled addressee in this case would be a team of that kind. Each medicinal chemist gave some evidence (in particular on receptor binding profiles) which I would normally have expected to come from the pharmacologist member of the team, but this was done about equally on each side, and no objection was taken. It did not represent a significant duplication, and it may, as Counsel for the Defendants submitted, just have been a symptom of a team whose members would need to cooperate particularly closely.
16. Alcon’s pharmacologist was Dr Achim Krauss. He was working in industry at Allergan at the priority date and has (and had then) direct knowledge of prostaglandins in the context of glaucoma.
17. Although clearly knowledgeable and good at explaining things, I found Dr Krauss to be a frustrating witness because he often gave long and non-responsive answers, repeatedly seeking to get across points he thought were important. Counsel for the Defendants showed great patience in dealing with this and did not submit that it was a symptom of a lack of independence, so I need say no more about it.
18. Dr Krauss also took an approach in his first report which I found odd, in that although his own expertise included, as I have said, prostaglandins, he gave evidence on the basis that the notional skilled pharmacologist was a generalist in glaucoma and would not know anything about them. He then dealt with the approach of a pharmacologist knowing of prostaglandins only in reply. I was assured (in post-trial written submissions) by Counsel for Alcon that this was not a tactical move, and accept that, and so I do not see any need to discount Dr Krauss’ evidence for this.
19. It was submitted by Counsel for the Defendants that Dr Krauss ought to have acknowledged in his written evidence that he was working with fluprostenol before the priority date. I agree that he should have but in itself it was a minor matter and probably just a facet of the way he addressed the skilled team, to which I have already referred.
20. Overall, the presentation of Dr Krauss’ evidence could have been better and more efficient, but was valuable to me and I have factored it into my analysis.
21. Alcon’s medicinal chemist was Dr David Cavalla. He has been a medicinal chemist in industry for many years. His evidence was extremely clear and cogent. He had no experience in prostaglandins or glaucoma at the priority date, but that expertise would come from the pharmacologist in the team and the skills of the medicinal chemist are largely transportable from one target to another.
22. The Defendants’ pharmacologist was Dr William Wilson. He was an academic at the priority date, with a research interest in the treatment of glaucoma, but with no experience in relation to the possible use of prostaglandin or its analogues.
23. Dr Wilson was in general a good witness. However, I did not think he had a very firm grasp of the concept of common general knowledge (“CGK”), which is not his fault - it is not an easy concept to the uninitiated. That plus his teaching background with its very wide scope led to his treating things as CGK too readily.
24. The Defendants’ medicinal chemist was Dr Sally Redshaw. Like Dr Cavalla, she worked in industry but not in prostaglandins or glaucoma. I found her extremely precise and clear in her answers and she was plainly doing her best to help the Court from a position of independence. However, although I have said above that it was forgivable for the medicinal chemists to give evidence which veered into the usual territory of the pharmacologist, I felt that in Dr Redshaw’s case this was accompanied by not tackling the medicinal chemistry content of Stjernschantz at all fully. There was no malice in this and it seems to have been a consequence of the way she was instructed, which also, in my view, was without malice and with the perfectly laudable aim of cutting down duplication. Nonetheless, the effect was that I do not think Dr Redshaw approached Stjernschantz in the way that an open-minded medicinal chemist without knowledge of the invention would have done. They would have read the whole thing and focused on all the medicinal chemistry content. I will have to return to this theme below.
25. It is well established that the Court’s task in assessing expert evidence in patent cases is not just to decide which expert witness in fact approximates most closely to the skilled addressee. Instead, what is important is the expert’s ability to see and explain things from the perspective of that notional person, and their reasons are particularly important. In the present case, I found Dr Krauss (even allowing for the issues mentioned above) and Dr Cavalla better able to do this than Dr Wilson and Dr Redshaw, and their reasons more convincing. One reason for this may be Dr Krauss’ real-world experience with prostaglandins, but I do not reach my conclusion merely because he had it and Dr Wilson did not, although that is one factor.
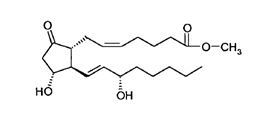
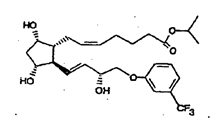
26. There was a major disagreement about the identity of the skilled addressee in the present case. It was common ground that the addressee would be a team; that it would include a medicinal chemist; and that it would include a pharmacologist. There was also no material dispute about the characteristics of the medicinal chemist.
27. However, the parties were far apart on the characteristics of the pharmacologist. It is a potentially important point because Alcon’s vision of the pharmacologist was aimed at cutting out key CGK, whereas the Defendants’ would both increase the available CGK and plant in the team’s mind the idea of using prostaglandin analogues for glaucoma.
28. The parties expressed their positions in different ways, but in a nutshell:
i) Alcon contended that the skilled pharmacologist had a general interest in finding new treatments for glaucoma; and further, since existing treatments did not include prostaglandins or prostaglandin analogues, the notional skilled pharmacologist would have no knowledge or interest in them.
ii) The Defendants contended that the skilled pharmacologist would be someone with an interest in using prostaglandins (or analogues) in the treatment of glaucoma.
29. The parties had different names for these rival, notional team member types: I will refer to the “glaucoma generalist” and the “prostaglandin specialist”, simply by way of convenient and meaningful shorthand.
30. The parties cited a number of authorities on the correct approach to this issue, but in my view there was nothing they referred to (apart from the EPO point mentioned below) that was not taken into account in the very detailed review by Birss LJ, in one of his last decisions at first instance, Illumina Cambridge Ltd. v. Latvia MGI Tech SIA and others [2021] EWHC 57 (Pat). I reproduce the whole of the relevant part:
58. Who is the person skilled in the art? Stated generally the law is clear that patents are directed to those likely to have a real and practical interest in the subject matter of the invention. This language is based on paragraph 81 on the judgment of Henry Carr J in Garmin v Philips [2019] EWHC 107 (Ch) in which the judge summarised the law in this area. The real practical interest in the subject matter includes devising the invention itself as well as putting it into practice and so, as was highlighted in Schlumberger v EMGS [2010] EWCA Civ 819, the concept of the person skilled in the art actually applies in two distinct circumstances. In a proper case they may be two different persons (or teams). One person skilled in the art is the person to whom the patent is addressed and whose attributes, skills and common general knowledge will be necessary to implement the patent. As Illumina submitted that person is always going to be the appropriate skilled team from the point of view of addressing sufficiency, since the patentee is entitled to put together his invention by combining any skill-sets he likes. As Pumfrey J said in Horne Engineering v Reliance Water Controls [2000] FSR 90 (quoted in Schlumberger at para 51):
“it is often possible to deduce the attributes which the skilled man must possess from the assumptions which the specification clearly makes about his abilities.”
59. The second kind of skilled person is the one relevant to obviousness. In nearly all cases they will be the same as the first kind (Schlumberger para 40) but Schlumberger was a case in which they were not, and that case illustrated why it would have been wrong to treat the two kinds as necessarily the same. The question then is what are the legal principles which define the identity of the second kind of skilled person.
60. One principle in Schlumberger was identified in paragraph 65:
“In the case of obviousness in view of the state of the art, a key question is generally “what problem was the patentee trying to solve?” That leads one in turn to consider the art in which the problem in fact lay. It is the notional team in that art which is the relevant team making up the person skilled in the art.”
61. This will be the governing approach in many cases but it can lead to trouble. There are cases of so called “problem-inventions” in which simply asking if the solution is obvious given the problem is unfair because inventiveness lay in identifying the problem. The fact the solution was obvious once you identify the problem does not prove a lack of inventive step in such a case. In fact experience shows that real cases are often more nuanced in that there can be aspects of a problem which are not common general knowledge and so one cannot always draw a sharp line between problem invention cases and other cases.
62. Furthermore, blindly applying an approach based on the definition of the problem to be solved could lead to a very narrowly defined skilled person and that can create its own difficulties, which were well described by Peter Prescott QC in Folding Attic Stairs v The Loft Stairs Company Ltd [2009] EWHC 1221 (Pat). He showed why it could be wrong to frame the art in a narrow way. At paragraphs 33-34 he said:
“33. Common general knowledge is quite different. It is what people skilled in the art actually do know, or ought to know, provided that knowledge is regarded as sound. Common general knowledge is not a phrase used in the Patents Act or the European Patent Convention. It would be difficult to define the person skilled in the art in this case, or the common general knowledge, because so far as I know there is no recognised profession or calling of designing folding attic stairways. At the date of the patent nobody seems to have done it in the British Isles except the Claimant and perhaps one other company. There must have been one or more companies in America, I suppose. It is unfair to define an art too narrowly, or else you could imagine absurd cases e.g. “the art of designing two-hole blue Venezuelan razor blades”, to paraphrase the late Mr T.A. Blanco White. Then you could attribute the “common general knowledge” to that small band of persons who made those products and say that their knowledge was “common general knowledge” in “the art”. That would have the impermissible result that any prior user no matter how obscure could be deemed to be common general knowledge, which is certainly not the law.
34. However it does not make much difference in this case, because the amount of special knowledge that is required to understand the patent in suit is not great. I would identify the person skilled in the art as one who has practical experience as a manufacturing carpenter, assisted by a metal fabricator. At the date of the patent (1996) this person or team would be vaguely aware of folding stairways in general terms, at most. The actual construction of old Stira, while known to many customers, was not common general knowledge in the art, in my judgment.”
63. So while Folding Attic Stairs neatly explains one of the difficulties, given its facts the judge did not have to identify a principle to be applied to solve it. Furthermore, while a too narrow definition could be unfair to the inventors, it could be just as wrong and unfair to the public to define a team so widely that their common general knowledge is so dilute as to make something seem less obvious than it really was (see Pumfrey J in Mayne v Debiopharm [2006] EWHC 1123 (Pat) at paras 3-4).
64. The other principled approach from Schlumberger to identifying the second kind of skilled person is to look at what is really going on in the art up to and at the priority date (Jacob LJ paragraph 42):
“I think one can draw from [Dyson v Hoover] that the Court, in considering the skills of the notional “person skilled in the art” for the purposes of obviousness will have regard to the reality of the position at the time. What the combined skills (and mind-sets) of real research teams in the art is what matters when one is constructing the notional research team to whom the invention must be obvious if the Patent is to be found invalid on this ground.”
65. This was summarised in Medimmune v Novartis [2012] EWCA Civ 1234 at paragraphs 76-77 as a principle that the court will have regard to the reality of the position at the time and the combined skills of real research teams in the art. In Medimmune the court found that “antibody engineering” was an established field by the priority date. There were 10 such real teams in the evidence and they were all likely to have a practical interest in the subject matter and to have the skills to implement it.
66. In the present case Illumina proposed, based on Medimmune, that a sensible test was to require something which could properly be called an established field at the priority date. Depending on the facts the field could be a research field as in Medimmune or a field of manufacture as in Folding Attic Stairs.
67. The advantage of this test is that it provides a principled way of solving the problem identified in Folding Attic Stairs. If the design and manufacture of folding attic stairs in particular was an established field then there is nothing unfair in defining the skilled person that way. But if not then the wider definition (general carpenter plus metal fabricator) is appropriate. In other words the width of the field in which the skilled person operates for the purposes of obviousness (aka the “art in which the problem lay” (per Schlumberger)) is ultimately governed by what was actually going on up to the priority date. It is not primarily a function of the invention itself, the problem to be solved, nor the patent’s text.
68. I conclude that in a case in which it is necessary to define the skilled person for the purposes of obviousness in a different way from the skilled person to whom the patent is addressed, the approach to take, bringing Schlumberger and Medimmune together, is:
i) To start by asking what problem does the invention aim to solve?
ii) That leads one in turn to consider what the established field which existed was, in which the problem in fact can be located.
iii) It is the notional person or team in that established field which is the relevant team making up the person skilled in the art.
69. Sub-paragraph (i) is phrased as it is rather than referring to a problem the patentee was trying to solve, because although those words are in Schlumberger, I do not believe the Jacob LJ was there intending to suggest that the identification of the problem is anything other than an objective exercise.
70. Sub-paragraph (ii) is phrased as it is for two reasons. First, there always will be some established field in which the problem would have been located. How wide the definition of that field should be will depend on the facts and what was going on in reality. Second, the field is the one in which the problem can be located, looking back from today as an exercise in hindsight. It does not matter at this stage if those in that field at the priority date did not perceive the particular problem or did not perceive it in the manner it is now characterised.
31. I intend to apply that approach. I take particular note of:
i) The requirements not to be unfair to the patentee by allowing an artificially narrow definition, or unfair to the public (and the defendant) by going so broad as to “dilute” the CGK. Thus, as Counsel for Alcon accepted, there is an element of value judgment in the assessment.
ii) The fact that I must consider the real situation at the priority date, and in particular what teams existed.
iii) The need to look for an “established field”, which might be a research field or a field of manufacture.
iv) The starting point is the identification of the problem that the invention aims to solve.
32. Counsel for Alcon referred to some EPO authority (T641/00 and the Case Law of the Boards of Appeal 9th Ed at 4.3.1). These relate to something slightly different, which is to the say the formulation of the objective technical problem for the problem-solution analysis. They have in common with the above approach to the skilled addressee the reminder to avoid hindsight in setting up the right question on obviousness.
33. Counsel for the Defendants relied on the following matters:
i) The existence of four commercial companies working on prostaglandin analogues for treating glaucoma, namely:
a) Allergan (where Dr Woodward was working, and whose group Dr Krauss had joined in 1989 to work on prostaglandins for glaucoma);
b) Alcon;
c) Pharmacia;
d) R-Tech Ueno.
ii) Papers indicating academic research groups working on prostaglandins and analogues for treating glaucoma (I give these by reference to the papers relied on by the Defendants in their closing skeleton):
a) Kerstetter et al., (1988), from the Mayo Medical School and the Department of Ophthalmology, Mayo Clinic and Foundation;
b) Camras et al., (1989), a collaboration between the Department of Ophthalmology, Mount Sinai School of Medicine and the Department of Ophthalmology, Columbia University;
c) Bito et al., (1989), a collaboration between Columbia University, Mt Sinai School of Medicine, the University of Florida and Pharmacia;
d) Kaufman and Crawford (1989), from the Department of Ophthalmology, University of Wisconsin;
e) Abstract no. 1153-69 by Camras et al., from ARVO 1993, a collaboration between the University of Nebraska Medical Centre and Creighton University;
f) Abstract no. 1158-74 by Rulo et al., from ARVO 1993, from the Glaucoma Centre of the University of Amsterdam, Netherlands Opthalmic Research Institute;
g) Abstract no. 2142-33 by Hotehama et al., from ARVO 1992, from the Department of Ophthalmology, Hiroshima University School of Medicine, Hiroshima, Japan;
h) Rácz et al., (1993), a collaboration between the Department of Ophthalmology, Markusovsky Hospital, Szombathely, Hungary and the Department of Ophthalmology, Columbia University; and
i) Giuffrè (1985), from the Ophthalmology Clinic, Palermo University.
iii) The 1989 book by Bito and Stjernschantz The Ocular Effects of Prostaglandins and Other Eicosanoids.
34. There is a risk of double counting with the academic papers, since e.g. Dr Camras recurs, and the Bito paper was a collaboration with Pharmacia. In addition, the papers to some extent are from groups of clinicians and not purely pharmacology and/or medicinal chemistry. I will take these points into account.
35. There was some discussion in the cross-examination of Dr Krauss of how many pharmacologists in industry were working in the field. He said 30 or so. I find it hard to assess whether this is many or a few, but it can at least be said to exceed a mere handful.
36. I consider that the Bito and Stjernschantz book was telling, in terms of the number of contributors (although unsurprisingly this overlaps with the other matters relied on), and also the way it characterised the field in its Preface:

……

37. This all paints a picture of the existence of a small, but quite well-defined research field. It was not a manufacturing field because although there were industry players they were still seeking to understand the biological systems they were interested in rather than making drugs (as I will explain below, some clinical trials were under way). They had reached the stage where their communal efforts had been collected into a book; the book was published in 1989 but publications had continued thereafter up until the priority date.
38. Those were the real facts on the ground. They have to be considered against the backdrop of the problem sought to be solved by the Patent.
39. In my view the problem (which I state in summary form because I do so in advance of explaining the CGK and Patent specification) was that while natural prostaglandins and the derivative PGF2a-IE had been found to be capable of lowering intraocular pressure, they had unacceptable side-effects that had brought a stop to efforts to use them. Synthetic analogues were needed to maintain the activity while avoiding the side effects.
40. Any solution to this problem would, in my view, lie in the field of the prostaglandin specialist.
41. I also take into account that there was little or no evidence that there were pharmacologists who really regarded themselves as glaucoma generalists. My impression was that ocular pharmacologists each tended actually to work in subspecialities, such as, for example, beta-blockers. Dr Wilson knew about all the subspecialities because he had teaching responsibilities, but that is a different matter.
42. I therefore agree with the Defendants in relation to the identity of the skilled addressee: the pharmacologist member of the team would be a prostaglandin specialist. The fact that in reaching this conclusion I have found that there was an “established field” must feed into my analysis of what was common general knowledge, but at the same time I must carry forward that the field was only barely and only recently an established one, that its practical accomplishments were limited, and that those working in it knew that there was much that they did not fully understand yet.
43. There was no dispute as to the applicable legal principles: to form part of the CGK, information must be generally known in the art, and regarded as a good basis for future action.
44. At my request the parties submitted a document setting out the agreed matters of CGK, which I have edited slightly:
45. Receptors are proteins typically located in cell membranes that specifically recognise and bind to ligands (i.e. smaller molecules (including drugs) that are capable of binding/‘ligating’ themselves to the receptor protein). The avidity of a drug to bind to a receptor is termed affinity. The efficacy of a drug is a measure of a drug’s ability to induce a response - the greater the maximal response, the more efficacious the drug. A drug which mediates a full response when bound to a receptor is a full agonist, a drug which mediates a partial response is a partial agonist and a drug which binds to a receptor and elicits no response is an antagonist.
46. The potency of a drug is a measure of the amount of drug required to elicit a particular response. Potency is often expressed as an EC50 value, meaning the molar concentration of a drug to achieve 50% of that drug’s maximal effect. The lower the EC50 value, the more potent the drug. Compounds with a high potency for a particular biological target are often attractive candidates for drug development because they can be therapeutically active at low concentrations reducing the risk of side effects caused by interactions between that drug and the biological targets responsible for side effects.
47. Generally, if a drug binds to a particular biological target with much higher affinity than to other biological targets, that drug is said to have high selectivity for that biological target. When a potent compound also has a high selectivity for the biological target responsible for efficacy it would be a prime candidate for drug development because the risk of side effects (caused by interactions with other biological targets) would be further reduced.
48. A prodrug is a pharmacologically inactive substance that is converted within the body into an active drug. Prodrugs are used in several scenarios but usually to improve the bioavailability of the active drug. In this context, often a prodrug is created to mask a charged, or polar, group which could impair the uptake of the molecule into the body. The chemical structure of the drug is therefore modified to create a prodrug to allow for example, the prodrug to pass through certain barriers in the body, such as lipophilic membranes. The concept of using prodrugs was well known in respect of topical drug delivery, including ocular drugs. A common method of preparing prodrugs to increase permeability through membranes is to form an ester of the parent drug.
49. The eye is composed of two segments: the anterior segment which is closest to the cornea and the posterior segment which is closest to the brain. The anterior segment comprises two connected chambers: the anterior chamber (between the cornea and the iris) and the posterior chamber (between the back of the iris and the posterior segment). Both the anterior and the posterior chambers contain aqueous humour a clear watery fluid. As well as providing the eye with the necessary nutrients the aqueous humour creates the intraocular pressure (IOP) that maintains the shape of the eye.
50. Aqueous humour is secreted continuously by ciliary epithelium, a single layer of cells covering the surface of the ciliary body which lies just behind the iris. It flows through the posterior chamber behind the iris, through the pupil into the anterior chamber. Aqueous humour drains out of the anterior chamber into the normal blood circulation via two main routes: the conventional outflow pathway and the uveoscleral pathway. In 1993 the conventional and uveoscleral outflow pathways were known to be the major and minor outflow pathways respectively. The conventional outflow pathway was pressure dependent, so as the IOP increases the outflow increases. The uveoscleral pathway is not pressure dependent (and this was known in 1993).
51. In August 1993 it was thought that the main cause of elevated IOP was reduced ability to drain aqueous humour due to blockage of the trabecular meshwork, resulting in an increased resistance in the conventional outflow pathway.
52. Glaucoma refers to a group of diseases characterised by optic nerve damage and associated progressive visual loss. The cause of glaucoma was poorly understood in 1993.
53. Glaucoma is usually (but not always) associated with an elevated IOP. The elevated IOP was associated with an increased resistance to the conventional outflow of aqueous humour. In 1993 elevated IOP was the most important, and the only treatable, risk factor for most types of glaucoma.
54. There was no cure for glaucoma in 1993. Glaucoma was typically treated by using medication(s) to lower a patient’s IOP. This was done by reducing the production of aqueous humour or increasing the outflow of aqueous humour.
55. Most treatments for glaucoma were administered topically as eye drops. However, rapid drainage from the eye (through the tear ducts) means that only a small amount (<10%) of the administered dose will permeate into the eye before being cleared from the ocular surface.
56. In 1993 patient compliance was a major issue with antiglaucoma medication for the following reasons:
i) the serious consequences of glaucoma were often not noticed by the patient until the disease had progressed significantly and so patients did not feel a need to take medication in the early stages;
ii) the unpleasant side-effects; and
iii) inconvenience of administration.
57. In 1993 various classes of drugs were available for the treatment of glaucoma:
i) Beta blockers (β-adrenergic antagonists): These were the most commonly prescribed and most effective glaucoma treatments. Beta blockers act by reducing the formation of aqueous humour, which in turn reduces IOP. In 1993 several topical beta blockers were available, of which timolol was considered the gold standard in terms of lowering IOP. Whilst their ocular side effects were not particularly serious, beta blockers could cause serious systemic side effects such as bronchospasm and cardiac issues.
ii) Carbonic anhydrase inhibitors (CAIs): These systemically-administered drugs also reduced the formation of aqueous humour, although they were not as effective as timolol in reducing IOP and the systemic side effects could be fatal.
iii) Miotics (or cholinergic (muscarinic) receptor agonists): These stimulate muscarinic receptors on smooth muscle in the eye increasing aqueous humour drainage through the conventional outflow pathway. They caused serious systemic side effects and ocular side effects (accommodative spasm, retinal detachment).
iv) Adrenergic agonists: Adrenaline (epinephrine) was also used to treat glaucoma. It was slightly less effective than timolol in reducing IOP and caused an array of ocular side effects as well as systemic cardiac side effects.
58. Timolol was the first line treatment for glaucoma in 1993. Where one drug was insufficient to achieve the required reduction in IOP, drugs were often prescribed in combination, particularly those with different mechanisms of action.
59. In 1993 there was a desire for new treatments for glaucoma. The ideal new drug for treating glaucoma would be more efficacious in lowering IOP than existing therapeutics with no or minimal side effects and a convenient dosage form and dosage regimen. However, no drug is ideal and in practice compromise would be necessary.
60. Prostaglandins are endogenous signalling molecules that are present throughout the body and involved in the regulation of many biological systems. They are produced from arachidonic acid (a fatty acid) and act locally near their site of synthesis and with a limited duration of action. There are different kinds of naturally occurring prostaglandins; the principal bioactive naturally occurring prostaglandins are PGE2, PGF2ɑ, PGI2, PGD2 and TxA2 (thromboxane).
61. Many of the naturally occurring prostaglandins comprise the same basic structure: 20 carbon atoms, including a five-membered ring, with an alpha chain and an omega chain. PGF2ɑ comprises 20 carbon atoms, which are arranged as two chains attached to a five-membered ring. The "alpha" chain consists of carbon atoms 1 to 7, and the "omega" chain consists of carbon atoms 13 to 20.
62. Naturally formed prostaglandins are single enantiomers i.e. the groups around the chiral carbons always have the same arrangement in space. PGF2ɑ has five chiral carbon centres.
63. By 1993 a significant body of research had been undertaken on the potential use of prostaglandins (and their antagonists) as drugs, particularly for cardiovascular and pulmonary diseases.
64. At lower doses, topical application of natural prostaglandins had been shown to lead to a reduction of IOP. Particular focus had been on PGF2ɑ which had been found in phase II clinical trials to effectively lower IOP in humans, albeit with clinically undesirable side effects. As at 1993, the prodrug PGF2ɑ isopropyl ester (“PGF2ɑ-IE”) had been found to be a very potent ocular hypotensive with particularly prolonged effects. The mechanism of action was not well known but the leading hypothesis was increased uveoscleral outflow.
65. Despite their IOP-lowering activity natural prostaglandins were not used as anti-glaucoma drugs in the clinic (other than in the context of clinical trials) due to their side effects, in particular ocular irritation (pain and so-called “foreign body sensation”) and conjunctival hyperemia (enlarged blood vessels - a vascular side effect). The ways in which ocular irritation was caused and conjunctival hyperemia arose were unknown.
66. By 1993, the skilled pharmacologist would have been well aware of the side effects in humans of PGF2ɑ-IE and would have known that these side effects had effectively ended any further clinical development of PGF2ɑ-IE as an anti-glaucoma medication for use in humans.
67. Although various animal models had been used to study the effects of potential ocular drugs (including prostaglandins), it was known that large variations existed in the ocular responses (both in terms of IOP reduction and side effects) of those species.
68. Non-human primates (including monkeys) were the closest animal model to humans, best reflecting the responses of man to topically applied ocular drugs. Dogs and cats were historically the most frequently used animal models for glaucoma. Glaucoma occurs naturally in beagles, providing a natural animal model. Cats were commonly used to assess IOP-lowering drugs in 1993 largely because they were readily available and easy to handle.
69. Conjunctival hyperemia is easier to detect in the eye of rabbits, dogs and guinea pigs than in cats and monkeys. Rabbits were especially commonly used to assess conjunctival hyperemia.
70. There is no satisfactory way of quantifying pain in animals. Observation of behaviour (blinking, prolonged eye closure and excessive tear secretion) can give clues, but pain is difficult to assess accurately. The experience of pain and irritation is subject to species variation. Cats were commonly used as a model for ocular irritation.
71. In 1993, there was no commercially available prostaglandin (or prostaglandin analogue) drug on the market for the treatment of glaucoma.
72. With their agreed statement of CGK, the parties identified the areas of disagreement on the CGK as lying in the following areas:
i) Prostaglandins as therapeutic agents to treat glaucoma;
ii) The receptor profile of prostaglandins;
iii) The proposed prostaglandin receptor classification systems;
iv) Fluprostenol (and its properties);
v) Latanoprost.
73. I found this too general to be fully helpful and asked for a further breakdown, which was provided. This also identified what Alcon accepted as to (i) information that could be identified from other documents and (ii) information that could be obtained from Stjernschantz. Alcon very justifiably made clear that those two categories were not accepted to be CGK, but their identification was helpful in seeing where and to what extent the disputes over CGK really mattered.
74. I found Alcon’s submissions on CGK to be rather extreme. For example, even on the assumption that the Defendants were right about the skilled addressee, Counsel for Alcon submitted that the pharmacologist member of the team would not have known that specific prostaglandin receptors had been identified, but only that natural prostaglandins acted at receptors of some sort. This degree of ignorance would not make sense; it amounts to saying that the skilled pharmacologist would not know the basic information available about how the systems in which they were interested worked.
75. This approach by Alcon was related to what I thought was an unrealistic view of how CGK had to be proved; it attacked the route put forward by the Defendants’ witnesses as to how each specific document would be found by the skilled pharmacologist. While I agree that Dr Wilson’s evidence could have been more thorough on this, and while he might have been rather dependent on what he was given by the Defendants’ advisers (for the perfectly understandable reason that he was retired and did not have ready access to materials), it is not necessary or realistic to prove exactly by what route the skilled addressee would find or access CGK, and it may well be enough to show that the information is contained in a number of well known reference-type works. I found this observation particularly relevant to the Coleman classification system.
76. I also reject Alcon’s approach of seeking to rule out general publications merely on the basis that they were general. For example, Rang & Dale’s Pharmacology (2nd Ed, 1991) was a general pharmacology work, and Shields Textbook of Glaucoma (3rd Ed, 1992) was a relatively general medical work about glaucoma, but that does not mean that the skilled addressee would not look to them, in addition to more specialist works, or that they could not form part of the evidence proving common general knowledge. In any event, the former referred to the Coleman classification, though both had a tone consistent with my finding above that the whole field was in an early stage of development.
77. I now turn to the disputed areas of CGK.
78. Although listed as an area of dispute by the parties, I think this was essentially agreed, in the event that Alcon was wrong about the skilled team. In any case, my findings are as follows, on the basis that the skilled pharmacologist member of the team was the Defendants’ specialist:
i) It was CGK that there was no authorised prostaglandin drug (natural or analogue) to treat glaucoma.
ii) It was CGK that latanoprost was a promising candidate for glaucoma treatment that had successfully completed phase II trials and entered phase III trials. My basis for this finding is that there were a number of publications on the topic, and that any skilled person would find out the current state of play, which latanoprost represented. This information was clearly spelled out in Stjernschantz in any event so, as was accepted, this dispute is of no great practical significance.
79. It bears repeating that it is common ground to have been CGK (see above) that natural prostaglandins had been found to have such significant side-effects in terms of irritation and hyperemia that they had been discarded as glaucoma treatments.
80. I will take the parties’ disputed topics ii) and iii) above together.
81. It is not in dispute that work had been done to study and classify prostaglandins, and prostaglandin receptors, and to characterise them.
82. There is also no doubt that there was a significant body of work on this front by Dr Coleman of Glaxo. It had been published in various books, papers and other reference publications. The argument is not about the existence of that material, but over whether it was CGK.
83. What Dr Coleman had done was to identify a number of prostaglandin receptor types, and to assign to each one or more specific ligands, i.e. a ligand with very high affinity for that receptor and much lower affinity for the other receptors.
84. For example a book chapter by Dr Coleman in 1987 contained the following:
85. The receptor for which PGF2a was the most potent natural ligand was called the FP receptor.
86. Dr Coleman also noted tissues thought to be rich in relevant receptors. For example, the cat eye iris is mentioned as being rich in FP receptors (and I agree with the Defendants that the cat iris model was known as a measure of FP receptor binding).
87. I agree that the Coleman classification system was CGK. The large number of references to it in both general and specialist works proved as much. I have mentioned Dr Coleman’s own work; other citations included Rang & Dale, and the “TIPS” Receptor Nomenclature Supplement put in evidence by Dr Wilson.
88. Saying that the Coleman classification system was CGK is, however, by no means the end of the inquiry. The skilled pharmacologist could not know of it without also knowing its purpose and its limitations.
89. The purpose of the system was classification and analysis. It was a means for organising information about receptors and ligands and studying them further. It was well-established as a good basis for that kind of action. It was not said to be established as a way of predicting (and certainly not with confidence) that a drug with a particular affinity for prostaglandin receptors would definitely work for a particular therapeutic purpose, still less what its side effects might be, or how they would be mediated. Nor did it have anything to do with whether a drug with a particular profile against prostaglandin receptors would also have other mechanisms of actions against other receptors.
90. As to its limitations:
i) It was not the only classification system. Another system from Dr Gardiner had been assimilated to it over time, but the system of Muallem remained live and was different.
ii) The whole exercise of studying the actions at the various prostaglandin receptors was hindered by the lack of any specific antagonists, to permit some of the receptor types to be blocked while others were studied.
iii) Only some of the receptors had been cloned; the FP receptor had not.
iv) It was not known whether the receptor types might have subtypes within them and if so what those might be. I touch on this further below.
v) Prostaglandin receptors were known to be widely spread around many organs in the body.
91. In short, the Coleman classification system was a work in progress.
92. The Coleman classification system is mentioned in Stjernschantz so again this issue, while hotly debated, may be of only minor importance; I return to this below.
93. The Defendants contend that fluprostenol was CGK. It is necessary to break matters down more than that.
94. It is true that fluprostenol is mentioned in some of the Coleman classification documents, as a highly potent FP-receptor agonist but which had very low, indeed essentially nil, agonist activity against the other receptor types with which Coleman was concerned. In other words, it was known to be very selective, and indeed more so than PGF2a. This profile made it highly relevant for classification, and so, for example, it is specifically mentioned in the TIPS supplement, and in Dr Coleman’s chapter in Hansch, Comprehensive Medicinal Chemistry (1st Ed, 1990) it is described as “diagnostic in establishing whether FP receptors exist in any particular tissue”.
95. So I agree that to this extent and for this purpose, fluprostenol was CGK, as part of the picture concerning the Coleman classification.
96. However, in terms of its previous use as a drug, fluprostenol had merely been a luteolytic agent in veterinary practice, which effect had been attributed to its FP receptor agonism. I do not think this would have been CGK to the skilled addressee working on glaucoma, but even if they had noticed it referred to in passing, it would not have been of practical interest.
97. Fluprostenol is not mentioned in Stjernschantz, which refers to PGF2a as the specific (natural) ligand for the FP receptor.
98. The Patent’s claims (which I address below) relate to the isopropyl ester of fluprostenol, which I have referred to above as FIE already. It is common ground that if the skilled team decided to test, or use, fluprostenol then it would be obvious to do so using the isopropyl ester, for reasons given above in relation to the CGK. So I need say no more about it, except that from time to time the parties’ representatives and their witnesses referred to fluprostenol when they probably strictly meant FIE. This was understandable and caused no problem; I have tried to be precise in this judgment but recognise that I may not have succeeded in every instance.
99. The patent begins with the following introductory paragraph:
[0001] The present invention relates to the treatment of glaucoma and ocular hypertension. In particular, the present invention relates to the use of fluprostenol isopropyl ester having the compound structure shown in Table 2 below to treat glaucoma and ocular hypertension wherein the dosage range for topical administration of fluprostenol isopropyl ester is between 0.05 and 10 micrograms per eye.
100. After then introducing the structures of PGF2a, cloprostenol and fluprostenol at [0002] and [0003], it describes the problem of the side effects from naturally occurring prostaglandins when used to lower IOP, and earlier attempts to address them, at [0004]:
[0004] Naturally-occurring prostaglandins are known to lower intraocular pressure (IOP) after topical ocular instillation, but generally cause inflammation, as well as surface irritation characterized by conjunctival hyperemia and edema. Many synthetic prostaglandins have been observed to lower intraocular pressure, but such compounds also produce the aforementioned side effects. Various methods have been used in attempting to overcome the ocular side effects associated with prostaglandins. Stjernschantz et al. (EP 364 417 A1) have synthesized derivatives or analogues of naturally occurring prostaglandins in order to design out selectively the undesired side effects while maintaining the IOP-lowering effect. Others, including Ueno et al. (EP 330 511 A2) and Wheeler (EP 435 682 A2) have tried complexing prostaglandins with various cyclodextrins.
101. It should be noted that the Stjernschantz patent application referred to, EP 364 417 A1, was document (3) in the TBA decision, but has different contents from the Stjernschantz article as pleaded in these proceedings.
102. The invention is then summarised at [0010] and [0011]:
[0010] It has now been unexpectedly found that fluprostenol isopropyl ester having the compound structure shown in Table 2 below and wherein the dosage range for topical administration of fluprostenol isopropyl ester is between 0.05 and 10 micrograms per eye shows significantly greater IOP reduction than the compounds of Stjernschantz et al., while having a similar or lower side effect profile. In particular, it appears that the addition of a trifluoromethyl group to the meta position on the phenoxy ring at the end of the omega chain provides a compound having excellent IOP reduction without the significant side effects found with other, closely related compounds.
[0011] In addition, it has also been unexpectedly found that fluprostenol isopropyl ester having the compound structure shown in Table 2 below and wherein the dosage range for topical administration of fluprostenol isopropyl ester is between 0.05 and 10 micrograms per eye is useful in treating glaucoma and ocular hypertension. In particular, topical application of ophthalmic compositions comprising this novel fluprostenol analogue results in significant IOP reduction.
103. There follows a long section about synthesis of the compounds of interest which is no longer of relevance.
104. Examples 5 to 9 then give information about the effects of certain compounds in animal models. The compounds are referred to as A to E. A is cloprostenol isopropyl ester, B is FIE, C and D are isopropyl esters of two of the compounds from the referenced Stjernschantz patent application, and E is latanoprost (which is an isopropyl ester).
105. Example 5 is a hyperemia test in a guinea pig model. Each of compounds A to E was tested, and the results are tabulated in Table A and shown graphically in Figure 1, which I found more accessible:

106. The results are summarised in [0065]:
[0065] Compound C (16-phenoxy-17,18,19,20-tetranor PGF2a, isopropyl ester) produces significant hyperemia at low doses, and at 0.3 and 1.0 mg doses, all eyes received one or more scores of +3. Compound D (17-phenyl-18,79,20- trinor PGF2a, isopropyl ester) produces less hyperemia than compound C, but significantly more than compound E (13,14-dihydro-17-phenyl-18,19,20-trinor PGF2a, isopropyl ester), which produces only mild hyperemia. The hyperemia produced by compound A (cloprostenol, isopropyl ester) and compound B (fluprostenol, isopropyl ester) appear to be intermediate between that of compound D and compound E, but this degree of hyperemia is also mild, and cannot be distinguished from that produced by compound E.
107. Example 5 does not directly compare FIE with PGF2a-IE but because it is shown to be indistinguishable in terms of hyperemia from latanoprost, and that in itself was known to be better than PGF2a-IE I consider that an indirect comparative conclusion can be drawn that FIE preserves the favourable hyperemic side effect profile of latanoprost over PGF2a-IE.
108. Example 6 involved testing compounds A to E for their IOP-lowering effects in cynomolgus monkeys. Results are shown in table 4 and table 5.
109. The data present a somewhat patchy picture because only one dose is used in table 4 and table 5 does not include FIE but only cloprostenol IE. In addition, latanoprost was found, for unexplained reasons, to be inactive at the single dose used in table 4.
110. However, in my view it is still possible, at least to the undemanding standard required for plausibility in the law of obviousness, to conclude that FIE as well as the other test compounds had a greater effect in lowering IOP than did latanoprost. Drs Wilson and Krauss appeared to agree about this in their written evidence.
111. Counsel for the Defendants sought in cross-examination to undermine whether such a conclusion was justified from tables 4 and 5, mainly on the basis of there being only a single dose used in table 4. However, as explained below the only plausibility attack (a squeeze) that was pleaded was about hyperemia and not IOP lowering. It would have been unfair to allow the attack on the IOP data without pleading, especially since it was only revealed after cross-examination of Dr Wilson had been concluded, and when I put that to Counsel for the Defendants he withdrew the point.
112. There is no use of FIE in examples 7 or 8 and I need say no more about them. Example 9 is merely some formulations and it does not matter.
113. Claim 1 of the Patent as upheld by the TBA is in the EPC 2000 form for second medical use claims and is:
A topical ophthalmic composition for use in the treatment of glaucoma and ocular hypertension comprising a therapeutically effective amount of fluprostenol isopropyl ester wherein the dosage range for topical administration of fluprostenol isopropyl ester is between 0.05 and 10 mg per eye
with the proviso that the composition does not include the following composition:
compound (F) 0.0001 wt%, fluprostenol isopropyl ester 0.001 wt%, benzalkonium chloride 0.01 wt%, dextran 70 0.1 wt%, disodium edetate 0.05 wt%, potassium chloride 0.12 wt%, sodium chloride 0.77 wt%, hydroxypropyl methyl cellulose 0.3 wt%, HCl and/or NaOH to adjust pH, and purified water q.s. to 100%, wherein compound (F) has the following formula:
and wherein fluprostenol isopropyl ester has the following formula:
114. The words from “with the proviso …” onwards can be ignored for the purpose of obviousness as they are a disclaimer to get over anticipation by EP 800. The dosage range is also not said to be relevant to obviousness (at least in this Court - it came into play in the EPO) and is very wide, so effectively the claim is a medication for use in treating glaucoma which contains an effective amount of FIE.
115. Claim 2 is a Swiss claim. Neither side says there is any difference from claim 1 for the purposes of these proceedings.
116. Conditional amendment 1 is set out below. The material point is that instead of the composition comprising a therapeutic amount of FIE, it must consist of it. This restricts the claim to FIE monotherapy and seeks to get around EP 800, which has FIE with another active, in a different way. With the amendments marked up, the conditionally amended claim is:
“1. A topical ophthalmic composition for use in the treatment of glaucoma and ocular hypertension wherein the composition consists of comprising a therapeutically effective amount of fluprostenol isopropyl ester administered as a solution, suspension, or emulsion in a suitable ophthalmic vehicle, and wherein the dosage range for topical administration of fluprostenol isopropyl ester is between 0.05 and 10 micrograms per eye, with the proviso that the composition does not include the following composition: compound (F) 0.0001 wt%, fluprostenol isopropyl ester 0.001 wt%, benzalkonium chloride 0.01 wt%, dextran 70 0.1 wt%, disodium edetate 0.05 wt%, potassium chloride 0.12 wt%, sodium chloride 0.77 wt%, hydroxypropyl methyl cellulose 0.3wt%, HCl and/or NaOH to adjust pH, and purified water q.s. to 100%, wherein compound (F) has the following formula:

and wherein fluprostenol isopropyl ester has the following formula:

117. The proposed amendments only seek to deal with novelty. They do not have any relevance to obviousness.
118. There was another proposed amended claims set but I do not need to deal with it, as it was to address an argument about extension of scope that fell away.
119. It is convenient at this point to address Alcon’s contentions as to the technical contribution of the Patent. There are said to be the following aspects to it:
i) A new and useful therapy for the treatment of glaucoma (of course this is on the assumption that the anticipation attack failed);
ii) A prostaglandin analogue which lowers IOP but without the hyperemia associated with PGF2a;
iii) A prostaglandin analogue which is better than latanoprost in terms of IOP lowering but with comparable good/acceptable hyperemic properties;
iv) A prostaglandin analogue with less hyperemia than the structurally closest analogue from the Stjernschantz 417 patent application identified in the Patent.
120. I did not see the relevance of the last of those points.
121. I have considered the factual basis of ii) and iii) above in reviewing the teaching of the Patent’s specification, and have accepted them. Point i) is also true, but it is important that it be taken with points ii) and iii) as a whole, because in total they mean that the technical contribution is a better glaucoma treatment (than latanoprost or PGF2a) and not merely an alternative one.
122. It was not argued by Alcon that the technical contribution lies in whole or in part in addressing the side effect of ocular irritation - that is not demonstrated by the Patent for FIE.
123. It was submitted for the Defendants that PGF2a (itself or as the isopropyl ester) was suitable “for use” in treating glaucoma. I said that I did not see the direct relevance of this, since there was and could be no case that PGF2a was within the claims. I understood Counsel for the Defendants to accept this, and further to accept that the side effects of PGF2a were recognised to be undesirable, and that improving side effects was part of the relevant motivation of the skilled person.
124. So I remain of the view that this issue of claim interpretation does not matter and does not arise, but in case I have misunderstood the matter, I will decide it.
125. Counsel for the Defendants accepted that there must come a point where side effects are so bad that a drug is not suitable for use despite having an effect on the disease state in question. In particular, he accepted that lethal side effects would prevent a drug being suitable for use. I think that the boundary must depend on context, the disease state including its severity, and the nature and effect of the side effects. So, for example, even the risk of death from side effects might not prevent a cancer chemotherapy from being suitable for use, whereas the same side effects would rule out a drug for, say, bad breath as suitable.
126. In the current context, glaucoma was regarded as an incurable, long-term condition. Neither ocular irritation nor hyperemia would be considered unbearable in the short term, but in the longer term they would be regarded as very burdensome, and were recognised as having a very material adverse effect on patient compliance. They were not just inconveniences.
127. I therefore consider that PGF2a was not suitable for use in treating glaucoma, and I am fortified in that view by the fact that those in the field considered it as being a dead-end, necessitating the search for other agonists with a better side effect profile.
128. I will deal with anticipation and then obviousness.
129. There was no dispute about the basic test - there has to be clear and unambiguous disclosure of all the features of the claim.
130. As I have said, anticipation was alleged over EP 800.
131. The Defendants’ argument over EP 800 was rather diffuse, and seemed to change through the trial. No objection was taken to this by Alcon and since the legal test for anticipation by a prior document depends purely on disclosure, there is no reason why the Defendants should not develop their contentions.
132. The only reference to FIE in EP 800 is in Example E, where it is used in combination with another, E series prostaglandin. However, Example E itself is excluded by the proviso to claim 1 as allowed by the TBA; Counsel for the Defendants accepted that, and even if it were not the case, proposed amended claim 1 is limited to monotherapy with FIE alone, as I have indicated above. Counsel for the Defendants accepted that too.
133. Since it was accepted that Example E in itself did not fall within claim 1, Counsel for the Defendants had to argue that there was a relevant disclosure elsewhere in EP 800. I was referred to the following parts of EP 800:
i) Page 5, which refers to isopropyl esters as being one of several preferred esters, but there is no reference there to fluprostenol.
ii) Formula (I), within whose scope FIE falls. But FIE is not individually described by it.
iii) The preferred compounds of Formula (I) on page 19. These include fluprostenol but not FIE.
134. I was not entirely clear if they were relied upon separately from Example E, or as modifying or expanding Example E. For example, paragraph 103 of the Defendants’ closing written submissions maintained the reference to the individualised disclosure of FIE in Example E.
135. The Defendants also submitted that the skilled addressee would not see the reference to the isopropyl ester form of fluprostenol being disclosed only in combination with the other components, but that it “comprises part of the general teaching”. I reject this; Example E is very specific and not a general teaching, and if other parts of the general teaching mentioned FIE then the Defendants would have relied on them.
136. That reference to the general teaching really exposes that the Defendants cannot show FIE being taught to the standard required for anticipation anywhere except in Example E (which is not within the claims for the reasons given above), but rather that they were putting forward a case of near-miss obviousness or an illegitimate, because undisclosed, combination of or modification of, Example E with or by other parts of the specification.
137. So the anticipation attack fails whether or not the claims are amended to monotherapy.
138. Alcon also relied on the principle that a second medical use claim can only be anticipated by a disclosure which makes plausible, so as to enable, the treatment effect claimed. There is, Alcon argued, no data in EP 800 to do this, and I agree that Dr Wilson accepted as much (Dr Krauss said the same).
139. Alcon majored on this point in its written closing submissions, along with extensive citation of authority for the legal proposition (e.g. Merck v. Ono [2015] EWHC 2973 at [173]-[175]). The Defendants made no response, and I think the point is well-founded, and another reason to reject the anticipation attack. I am a little puzzled why the point did not prevail before the TBA; it may not have been argued. If the TBA had considered it and rejected it I would want to understand why, but that is not the position.
140. The claims also require a particular dose range. If the Defendants’ attack had otherwise been successful then this on its own would not have saved the claims of the Patent since the range is very wide and overlaps with what is taught in EP 800.
141. There was no dispute about the basic principles. I was referred to the decision of the Supreme Court in Actavis v. ICOS [2019] UKSC at [52] - [73], with its endorsement at [62] of the statement of Kitchin J as he then was in Generics v. Lundbeck [2007] EWHC 1040 (Pat) at [72].
142. As I have already said, Stjernschantz was published in 1992 in Drugs of the Future, and its title is “Phenyl substituted prostaglandin analogs for glaucoma treatment”. Its authors are Drs Stjernschantz and Resul from Pharmacia, in Sweden.
143. Stjernschantz has a 2-page introduction: after a general discussion about earlier misconceptions concerning the role of prostaglandins in inflammation in the eye, and aspects of the available animal models, there is a reference to the established effect of PGF2a (and its ester) in lowering IOP, but with the side effects of irritation and hyperemia. On my findings as to the skilled team, this would all be CGK.
144. There is then a long section about general methods for synthesis of phenyl substituted PGF2a analogues. The syntheses are not relevant to this judgment, save to note that in the compounds of Scheme 2 where part of the omega chain is substituted with a phenyl ring, latanoprost is compound 8, and its racemate is compound 11; compound 5 is also referred to later as highly active.
145. There is then a structure activity relationship analysis of the Scheme 2 compounds. As shown by Figure 2, they all had marked and dose-dependent miotic effect in a cat eye model. Table I sets out their irritative and hyperemic effect in the cat’s eye, with a total lack of irritation, but some hyperemia.
146. At page 695, in a passage bridging the two columns, the authors wrote:
In spite of the fact that PGF2alpha-ie is very irritative in the cat eye, none of the phenyl substituted PGF2alpha-ie analogues caused any ocular irritation as judged from the behavior of the animals as well as from the degree of lid closure after topical administration of the compunds (Table I). The marked miotic effect of these compounds in combination with the total lack of irritative effect strongly suggests that substitution of part of the omega chain with an aromatic ring structure either causes conformational alteration in the molecule or imposes a steric hindrance, which enables a discrimination between different prostaglandin receptor subtypes.
147. In this context, I consider that the reference to “prostaglandin receptor subtypes” is to the receptors listed in the left-hand column of table III, but that does not mean that the skilled team would have thought that there were not subtypes of those types. That was an unknown. Another 1993 publication by Resul (cited as prior art but later dropped) used receptor subtypes in the latter sense, and while I do not find that that paper was CGK in itself, I do find that it reflected the CGK understanding that it was not known what subtypes there might be.
148. The IOP effect of the Scheme 2 compounds was then tested in a monkey model, with results shown in Figure 3, where compounds 5, 8 and 11 were particularly active, to much the same degree as PGF2a-ie.
149. At page 696 in the right hand column there is the following comment:
Repeated administration of compound 11 in laser treated ocular hypertensive monkeys caused a sustained reduction in IOP throughout the treatment period (51). Surprisingly, even if these analogues reduce IOP in monkeys they have very little effect on the IOP in cats (28) or rabbits (unpublished results).
150. This comment is relevant to an argument made by Alcon that a paper by Woodward in IOVS (put in by Alcon for cross-examination, not in the case earlier) entitled “Prostaglandin F2a Effects on Intraocular Pressure Negatively Correlate with FP-Receptor Stimulation” reports that fluprostenol was inactive in lowering IOP in rabbit and cat models (so a test in those models would put the skilled team off fluprostenol even if they got to testing it). The Defendants’ response was that since Stjernschantz says essentially the same thing being the case (“surprisingly”), the point cannot have any relevance to obviousness. I agree with the Defendants on this, but the point was really a sideshow, save that it does show the complex and confusing picture in relation to animal models in this field.
151. At the end of this section, in the left hand column on page 698, the authors wrote;
As can be seen in Table I, all phenyl substituted PGF2alpha-ie analogues (Scheme 2) induced clearly less hyperemia than PGF2alpha-ie. The analogues exhibiting least conjunctival hyperemia were generally those exhibiting least pharmacologic activity such as the earlier mentioned 15-OH epimers 6, 9 and the 15-keto 7,10 17-phenyl substituted prostaglandin analogues (Table I).
152. I agree with Alcon that this gives the impression that hyperemia was in some way correlated to activity. It would reduce any confidence that the skilled addressee could otherwise have that it would be possible to achieve good activity (matching latanoprost, for example), while reducing or eliminating hyperemia.
153. Starting later on page 698 there is a section about latanoprost. Table III gives its prostaglandin receptor profile against the FP, EP1, EP2, EP3 DP/IP and TP receptors. For each receptor, the EC-50 of latanoprost is given, and under the heading “Specific ligand” the EC-50 for the relevant natural prostaglandin or an analogue is given. Latanoprost has much lower affinity for the receptors other than FP (but among those, slightly higher for EP1).
154. The same paragraph that introduces Table III points explicitly to the Coleman classification system (reference (56)). I consider that even if the skilled addressee did not know about the Coleman classification as a matter of CGK they would certainly look it up so as to understand this part of the teaching of Stjernschantz. I am not saying that they would look up all the references in the paper by any means (there are 61), but unless they acquainted themselves with the Coleman classification then I do not think they could follow Table III, which is plainly material teaching about latanoprost. For these reasons, I think the dispute about whether the Coleman classification system was CGK is not very important, although it might be said that the skilled addressee would feel more at home with the teaching if they already knew to what reference was being made.
155. Details are then given for some clinical trials on latanoprost and compound 11.
156. Starting from page 700, the authors looked at the effect of variation of length of the phenyl substituted omega ring. Figure 4 shows the miotic and irritative effects of various analogues, with compound 5 showing a very good miotic effect with minimal irritation.
157. In the right hand column on page 700, the authors wrote the following:
These results indicate that the 17-phenyl-18,19,20-trinor- PGF2alpha-ie is unique in that this compound exhibits a structural conformation with no affinity for PG receptors involved in the sensory irritative response (presumably PG receptors on sensory nerves), while retaining the affinity for FP receptors as demonstrated by the miotic response. In contrast, PGF2alpha-ie analogues with shorter or longer phenyl substituted omega chain did show some affinity for PG receptors mediating nociceptive impulses. However, this affinity was much weaker than that of PGF2alpha-ie. It appears that the steric hindrance of the phenyl ring and the interatomic distances between functional groups in the molecules are important for drug-receptor interaction.
158. There was a debate in the evidence before me about this. The disagreement between the experts was as to the confidence that could appropriately be given to the statement that it was prostaglandin receptors, particularly on the sensory nerves, that were responsible for the irritative response. In my view, the skilled team would not take this statement as one of scientific fact and would assess it for what it was worth. They would think it was being put forward as a hypothesis (as the Defendants accepted, and as the word “presumably” indicates). They would note that there was no experimental proof that the side effects were all mediated through prostaglandin receptors alone.
159. Starting on page 701 there is then a section looking at “Effects of substituents on the phenyl ring”. Certain changes were observed to leave unaltered, or alternatively to greatly reduce, miotic activity relative to PGF2a-IE and compound 5. Nearly all the analogues showed no irritative effect.
160. In a passage bridging pages 701-702 the authors commented on “Importance of ring structure on the omega chain” as follows:
From what is mentioned above, it is evident that by substituting part of the omega chain of PGF2alpha-ie with a phenyl ring, it is possible to totally eliminate the ocular irritating effect and to markedly reduce the hyperemic effect of PGF2alpha-ie. Although a phenyl ring substitution seems to be particularly beneficial, substitution with other ring structures such as cyclohexyl, thiophene and biphenyl also yields compounds with distinctly better side effect profile than that of PGF2alpha and its prodrugs in the eye (unpublished results). Thus, PGF2alpha possessing a terminal ring moiety on the omega chain exhibits a markedly improved therapeutic index in the eye.
161. Then the authors commented that they were studying other phenyl substituted analogues, and set out their conclusions at page 702:
PGF2alpha and its isopropyl ester have been shown to be potent ocular hypotensive agents in several animal species and in man. However, the frequent and disturbing side effects in the eye make it impossible to utilize PGF2alpha as an ocular hypotensive agent clinically. Whereas the prodrug esters of PGF2alpha do not significantly reduce the adverse effects in the eye, partial substitution of the omega chain with a phenyl ring dramatically reduces the ocular side effects of PGF2alpha-ie. Such substitution totally eliminates the superficial irritating effect of PGF2alpha-ie in the eye. This is probably due to a conformational change of the omega chain in the prostaglandin molecule, or steric hindrance, which enables a discrimination between different prostaglandin receptor subtypes. The most optimal chain length to which the ring structure is attached seems to be 5 carbon atoms (17-phenyl-18,19,20-trinor). The biologic activity of these compounds may further be altered by substitutions in the phenyl ring.
One of the most promising analogues 8 latanoprost is presently undergoing phase II clinical testing with encouraging results. This drug has been shown to potently reduce IOP in glaucoma patients with few side effects.
162. The first part of this tracks the CGK and the second part summarises at a very high level the work that I have set out above. As previously, the skilled addressee would read the discussion of the effect of the substitutions as a hypothesis, and would not think that all the observations could necessarily be explained by reference to effects at prostaglandin receptors alone.
163. There was a debate between the parties as to whether Stjernschantz was a significant publication or not. I agree with the Defendants that it was of appreciable note at the time, and I reject Dr Krauss’ perspective that it was of no significance and/or that Dr Stjernschantz himself was not known in the field. Dr Stjernschantz had participated in significant publications, and Dr Krauss accepted that he has personally following Dr Stjernschantz’s work closely himself. I was also struck by the Alm paper in 1993 “The potential of prostaglandin derivates in glaucoma therapy” which noted Stjernschantz as a paper of outstanding interest.
164. The Defendants also relied on the fact that IUPHAR still today cites Stjernschantz in support of the proposition that “FP receptor stimulation also profoundly lowers intraocular pressure in laboratory animal species and man”. However, this cannot help much with how the paper was seen at the time, and the top-level summary of what the paper did show, it does not really help the Defendants as it makes no reference to side effects or how they might be mediated.
165. I will apply the Pozzoli analysis.
166. As to Pozzoli questions 1 and 2, I have identified the skilled team and the common general knowledge above.
167. As to Pozzoli question 3, the parties characterised the difference(s) between Stjernschantz and the claims of the Patent in very different ways.
168. Alcon characterised there as being three differences, by reference to the structures of latanoprost (which it says was the most promising of the compounds in Stjernschantz) and of FIE, as follows:

169. The Defendants, on the other hand, expressed the difference as being merely the choice of a different selective FP receptor agonist, i.e. FIE.
170. This was more than merely a presentational issue of the patentee seeking to maximise the number of differences and the defendant trying to minimise it. The disparity in the way the parties characterised the differences was more substantive than that and related to the nature of the skilled team and the way they would see the work reported in Stjernschantz. Alcon’s position had a much greater focus on the medicinal chemistry content of Stjernschantz and its SAR work, and that is why it described the differences in terms of structure. I return to this below. My conclusion is that Alcon’s approach better reflects the contents and approach of Stjernschantz and the presence of a medicinal chemist as an active member of the skilled team.
171. In any event, with the skilled team and the CGK being as I have identified above, the task for the Defendants was to show that it was obvious to use FIE, instead of any of the analogues in Stjernschantz, to treat glaucoma.
172. The Defendants’ case was that Stjernschantz showed that it was most probably FP receptor binding that was responsible for reduced IOP, while side-effects were mediated by other prostaglandin receptors, and that once that was known, it would be obvious to try FIE for treating glaucoma, because it was known from the CGK to be a potent and selective FP receptor agonist. Thus, they argued, FIE fit the profile of an efficacious, side-effect sparing prostaglandin analogue.
173. As I understood it, it was also the Defendants’ argument that the question of side-effects ought not to enter the analysis on obviousness, since, they said, the Patent does not make plausible any reduction in hyperemia relative to PGF2a, or, indeed, any improvement in lowering IOP compared with latanoprost (or the other compounds of Stjernschantz). However I have found on the facts when dealing with the Patent’s teaching that a reduction in hyperemia is made plausible, and that the Defendants’ attack on the plausibility of IOP lowering was dropped, and anyway inconsistent with the written evidence going into trial.
174. Therefore, my consideration of obviousness ought to include, to an appropriate degree, consideration of those matters.
175. The Defendants’ case is a simple one, and Alcon focused in on that to argue that it is really an attack over the common general knowledge alone. Such attacks, Alcon argued, have to be scrutinised with care because they are a way of crafting an obviousness argument without due regard to the real details of actual work in a concrete context.
176. I disagree with Alcon on this; the Defendants were clearly building their attack over Stjernschantz, which contained what was an important building-block in their argument, namely the concrete identification of the FP receptor’s involvement. However, I agree with Alcon in a more general sense that the Defendants’ attack was developed at a considerably greater level of simplicity and generality than either Stjernschantz or the CGK justified, and did not address adequately a number of more detailed, practical points.
177. I think the following factors are particularly important:
i) The field, as I have identified it in addressing the skilled addressee was at a very early stage and there were many uncertainties in it.
ii) The field had hit a barrier with the failure of PGF2a because of side effects.
iii) The field was just starting to recover from that with the development of latanoprost.
iv) Stjernschantz reports encouraging early results with latanoprost, but solid data was still awaited.
v) Stjernschantz reports a theory about the cause of side effects but without proof; Stjernschantz focuses on prostaglandins and their receptors, but does not contain a demonstration that the side effects experienced were a result of action at prostaglandin receptors alone.
vi) Although Stjernschantz’s results in relation to the side effect of irritation were very good, the position on hyperemia was much less clear and there was an indication that the better the effect on IOP the greater the hyperemia. It was hard to see how to progress from there.
178. These issues all would mean that once the skilled addressee looked into the detail of Stjernschantz, their confidence that progress could be made with improving or maintaining IOP reduction while reducing hyperemia would be very limited.
179. I also think it is important to have in mind the kind of paper that Stjernschantz is, and the kind of work it presents. It includes aspects of pharmacology and aspects of medicinal chemistry, and both are very important. The overall teaching is of using a phenyl ring as a substituent in the omega chain, and there are three sections looking at the structure activity relationships of specific types of changes within that broad description; I have listed them above.
180. However, Stjernschantz does not mention fluprostenol at all. When it gives the EC-50 values for latanoprost in table III it makes a comparison using, in the main and for the FP receptor, the natural ligand. It does not use fluprostenol.
181. In my view, the natural way for the skilled team to approach obvious developments from Stjernschantz would be to consider further prostaglandin analogues, altered in ways concretely reasoned out from the structure activity work described. This would be logical and routine and in keeping with the approach of the paper. It is suggested in the penultimate paragraph of the paper.
182. In practical terms the skilled team might well wait and see what the latanoprost results from the phase III trials were, but that does not alter what the general approach would be.
183. I must not overlook that more than one approach may be obvious from a piece of prior art. In principle it could be obvious both to make structural changes based on Stjernschantz’s structure activity relationship work and also obvious to work with compounds with significantly different structures but similar patterns of activity. It depends on the facts, but Stjernschantz’s being a paper with such a strong basis in structure activity relationships is a point against trying other compounds based on activity.
184. It was very clearly the Defendants’ case that FIE was obvious to try based on its affinity and selectivity for the FP receptor and not structure (there was a slight attempt in their closing submissions to rely on the fact that fluprostenol has a phenyl group at the end of the omega chain, but this was unconvincing because it was couched at a much higher level of generality than how Stjernschantz expresses matters, and had not really been pursued in the evidence).
185. This difference is, as I have said the reason for the parties’ different approach to the third Pozzoli question.
186. In my view, the Defendants’ attack has the following significant problems:
i) The CGK of fluprostenol was as an analytical tool, not a medicine.
ii) Insofar as fluprostenol was known as a medicine, it had achieved use only in a very different field (luteolysis in animals).
iii) Fluprostenol is not mentioned in Stjernschantz (as I have already said).
iv) No case was made that fluprostenol would be specifically identified as consistent with the structure activity relationship work in Stjernschantz.
v) Efforts with fluprostenol might be accompanied by the hope that it would give better IOP lowering and/or reduced side effects, but there could be no positive expectation. And in particular, it would be thought, for reasons explained above, that a better IOP lowering effect would be accompanied by more hyperemia.
187. The last point bears some expansion: motivation and expectation of success may be important factors when it comes to obviousness, and their relative importance depends on the context and the facts, but a hope for a positive result sufficient to justify research being done does not necessarily imply an expectation of success (see e.g. MedImmune v. Novartis [2012] EWCA Civ 1234 at [90]-[91], referred to in Actavis v. ICOS). Even had the skilled team thought of trying fluprostenol in animal models to assess its possible use to treat glaucoma, they would have regarded it as very uncertain what effect it would have on side effects. Even if they had thought (which I do not think was established) that initial animal experiments with fluprostenol would have needed such low resources that it could be justified as a gamble, that would not make it obvious.
188. But in any event, bearing points i) to iii) in mind in the context of the overall direction of Stjernschantz, I do not think the skilled team would without invention have turned its mind to fluprostenol as a possible treatment in the first place. Even if it did, the prospects of success (having regard to efficacy and side effects) would be very uncertain. Much more attractive options consistent with the overall teaching and direction of Stjernschantz were available.
189. The Defendants relied on the evidence of Drs Wilson and Redshaw, and on various answers given by Drs Krauss and Cavalla in cross-examination.
190. Dr Wilson’s evidence was that Stjernschantz would motivate the skilled addressee to test other potent and selective FP agonists as alternative compounds for use in the treatment of glaucoma, and knowing of fluprostenol and its characteristics, that would have been the first choice. He did recognise that it would also be possible to make analogues based on the SAR work, but said that would have been less attractive, for reasons to be given by Dr Redshaw.
191. This did not persuade me. In particular, I thought it failed to deal with why the skilled team would think of fluprostenol in the first place, was almost entirely lacking in analysis of the prospects of success to be expected, and did not take account of the nature of the work done, and suggestions by, Stjernschantz.
192. Dr Redshaw considered Stjernschantz only rather briefly. She picked up the analysis only at page 701, dealing with substituents on the phenyl ring. Based on that, she suggested that the skilled team would look to compounds with high affinity and selectivity for FP receptors, and would not be guided by, or limited to, what the SAR work in Stjernschantz supported. Indeed, she was quite critical of this part of Stjernschantz’s work, which seemed to me rather inconsistent with the Defendants’ overall case that Stjernschantz was a high quality publication, recognised as such.
193. I found her analysis very limited and not a good basis for the proposition that the skilled addressee would just switch to other, quite different compounds. I did not think it properly reflected how a medicinal chemist would approach Stjernschantz; they would read the whole of it carefully and suggest specific, rational, structural changes based on the SAR work done.
194. As I have mentioned, the Defendants also relied on answers given in cross-examination. I think it is particularly important in the present case to read the whole passages. When that is done, I think the answers given were not really supportive of the Defendants’ case, and certainly not enough to undermine the evidence that Alcon’s experts had given in their written reports, which in general they continued to support and which I preferred over that of the Defendants’ experts.
195. The two particular extracts from the oral evidence relied on in the Defendants’ written closing submissions were:
i) At T3/424 Dr Cavalla was asked whether fluprostenol would be “on your list”, and he said that he was not saying that it would not. The Defendants deployed this as an acceptance that fluprostenol would be “on the list”. But Dr Cavalla went on to point out that what would happen with fluprostenol was unknown and untested and limited his statement to it “possibly” being on the list.
ii) Similarly, answers given by Dr Krauss at T3/550-551 were relied on as being an acceptance that if the data in table III of Stjernschantz were taken at face value then testing fluprostenol was obvious. But the answers were also on the assumption, which he had not accepted, that the relevant side effects were entirely down to activity at FP receptors. Even leaving that aside, his acceptance of what was put was limited.
196. Taking all these matters together I reject the obviousness attack over Stjernschantz. I have done so on the basis of the Defendants’ view of the skilled team. The Defendants’ case would only have been more difficult on Alcon’s view of the skilled team.
197. The pleaded point was as follows:
“3. In the event that the claims of the Patent are not obvious because it was understood that the administration of PGF2a isopropyl ester would cause ocular inflammation and irritation, the Patent fails to make plausible that the compounds of the invention are suitable for use in treating glaucoma and ocular hypertension. In particular, the data in the specification of the Patent fails to evidence an improvement in these side effects when the compounds of the invention are used over PGF2a isopropyl ester.”
198. “Inflammation” was changed to “hyperemia” by an amendment made during trial when it was pointed out by Alcon that the two are not necessarily synonymous. But both sides had been proceeding in their expert evidence on the basis that the squeeze was alleged to operate in relation to hyperemia, so I allowed the amendment, over Alcon’s objection.
199. The point fails on the facts, however, since, as I have explained above, the Patent does make it plausible that FIE causes reduced hyperemia compared to PGF2a.
200. The pleading does not allege that the Patent does not make plausible a better effect on IOP lowering. I have mentioned this above, and that the Defendants did not press the argument.
201. Overall therefore the squeeze made no difference and does not help the Defendants.
202. My conclusions are:
i) The allegation of anticipation by EP 800 fails.
ii) The allegation of obviousness over Stjernschantz fails.
iii) The Patent is valid.
iv) There is no need for any amendment.
203. I will hear Counsel as to the form of Order if it cannot be agreed. I direct that time for seeking permission to appeal shall not run until after the hearing on the form of Order (or the making of such Order if it is agreed).










