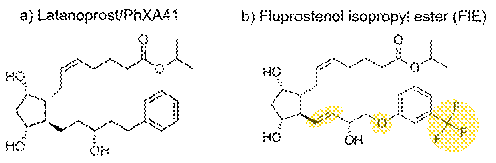This judgment was handed down by the Court remotely by circulation to the parties' representatives by email and release to The National Archives. The date and time for hand-down is deemed to be 10:30 on 28 June 2022.
Lord Justice Arnold:
Introduction
- The Claimants (“Alcon”) were respectively the proprietor and the exclusive licensee of European Patent (UK) No. 1 920 764 (“the Patent”) until it expired on 3 August 2014 and of Supplementary Protection Certificate SPC/GB12/038 (“the SPC”) based on the Patent until the SPC expired on 28 May 2017. The Patent concerns the use of fluprostenol isopropyl ester (“FIE”), a prostaglandin F2α (“PGF2α”) analogue also known as travoprost, for the treatment of glaucoma and ocular hypertension. The priority date of the Patent is 3 August 1993.
- When this claim was issued on 14 July 2014 the Defendants were preparing to market generic travoprost eye drops for the treatment of glaucoma and ocular hypertension. Alcon applied for an interim injunction to restrain the Defendants from launching their product which was granted by consent with the usual cross-undertaking in damages by Alcon. The Defendants did not dispute infringement, but contended that the Patent, and hence the SPC, were invalid. Due to protracted but unsuccessful negotiations between the parties the claim did not come to trial until March 2021, by which time both the Patent and the SPC had long since expired, but Alcon’s potential liability under the cross-undertaking remained.
- It is very unusual for the Patents Court to have to try a patent case where the priority date of the patent in suit is as long as 27½ years in the past. Furthermore, the lengthy gestation of the case caused the expert witnesses some difficulty in the preparation of their reports. These factors have no bearing on the legal issues in the case, but they mean that the dangers of hindsight are even more acute than they commonly are in patent cases.
- The Defendants contended that the Patent was invalid on three principal grounds: (i) lack of novelty over European Patent Application No. 0 603 800; (ii) obviousness over J. Stjernschantz and B. Resul, “Phenyl substituted prostaglandin analogs for glaucoma”, Drugs of the Future, 17(8), 691-704 (August 1992) (“Stjernschantz”); and (iii) insufficiency, but only as a “squeeze” on obviousness. Meade J rejected all three grounds for the reasons given in his judgment dated 23 April 2021 [2021] EWHC 1026 (Pat). The Second Defendant (“Aspire”) now appeals, with permission granted by myself, on the issues of obviousness and insufficiency.
The skilled team
- It was common ground before the judge that the Patent was addressed to a skilled team consisting of a pharmacologist and a medicinal chemist, but there was a significant dispute as to the attributes of the pharmacologist. The judge found at [27]-[42] that, as the Defendants contended, the pharmacologist would be someone with an interest in using prostaglandins (or analogues) in the treatment of glaucoma (a “prostaglandin specialist”). (For brevity the judge dispensed with referring to ocular hypertension as well as glaucoma, and I shall follow his example).
- The judge made no finding as to the relationship between the two members of the team. Aspire criticises this omission, and I shall have to return to it when considering the appeal on obviousness.
The expert witnesses
- Each side called two expert witnesses, a pharmacologist and a medicinal chemist. Alcon’s pharmacologist was Dr Achim Krauss and Alcon’s medicinal chemist was Dr David Cavalla. The Defendants’ pharmacologist was Dr William Wilson and the Defendants’ medicinal chemist was Dr Sally Redshaw. The judge considered that all four experts had done their best to assist the court, but he noted two features of their evidence that are relevant to the appeal.
- First, he noted at [18] that Dr Krauss “took an approach in his first report which I found odd, in that although his own expertise included … prostaglandins, he gave evidence on the basis that the notional skilled pharmacologist was a generalist in glaucoma and would not know anything about them” and “dealt with the approach of a pharmacologist knowing of prostaglandins only in reply”. Nevertheless the judge did not consider that this adversely affected the weight to be given to Dr Krauss’ evidence.
- Secondly, the judge noted at [24] that, as consequence of the way in which she was instructed (which had the laudable aim of avoiding duplication), Dr Redshaw did not “tackl[e] the medicinal chemistry content of Stjernschantz at all fully”. As a result, Dr Redshaw had not:
“approached Stjernschantz in the way that an open-minded medicinal chemist without knowledge of the invention would have done. They would have read the whole thing and focused on all the medicinal chemistry content.”
Common general knowledge
- The judge set out at [44]-[71] various matters which the parties were agreed were common general knowledge to one or other member of the skilled team. At [72]-[98] the judge made findings concerning five topics as to which there was a dispute. The agreed matters and findings that are relevant to the appeal may be summarised as follows.
General pharmacological principles
- Compounds with a high potency for a particular biological target are often attractive candidates for drug development because they can be therapeutically active at low concentrations, reducing the risk of side effects caused by interactions between that drug and the biological targets responsible for side effects.
- Generally, if a drug binds to a particular biological target with much higher affinity than to other biological targets, that drug is said to have high selectivity for that biological target. When a potent compound also has a high selectivity for the biological target responsible for efficacy it would be a prime candidate for drug development because the risk of side effects (caused by interactions with other biological targets) would be further reduced.
Prodrugs
- A prodrug is a pharmacologically inactive substance that is converted within the body into an active drug. Prodrugs are used in several scenarios, but usually to improve the bioavailability of the active drug. The chemical structure of the drug is modified to create a prodrug, for example, to allow the prodrug to pass through certain barriers in the body, such as lipophilic membranes. A common method of preparing prodrugs to increase permeability through membranes is to form an ester of the parent drug.
Glaucoma
- Glaucoma is a disease (more strictly, a group of diseases) of the eye characterised by progressive visual loss. It is usually associated with an elevated intra-ocular pressure (IOP), which was a known risk factor for glaucoma in 1993.
Treatment of glaucoma
- In 1993 glaucoma was typically treated by using medications aiming to reduce the patient’s IOP. Most treatments were administered topically as eye drops.
- Patient compliance was a major issue with antiglaucoma medication for various reasons, including the unpleasant side effects. The main classes of drug available for the treatment of glaucoma were beta-blockers, carbonic anhydrase inhibitors, miotics and adrenergic agonists. The first line treatment for glaucoma in 1993 was timolol (a beta-blocker). Where one drug was insufficient to achieve the required reduction in IOP, drugs were often used in combination. There was a desire for better drugs, which were more efficacious in lowering IOP with no or minimal side effects.
Prostaglandins
- Prostaglandins are endogenous signalling molecules present throughout the body. By 1993, they had been the subject of a significant body of research for potential use as drugs, particularly for cardiovascular and pulmonary diseases.
Prostaglandins and the treatment of glaucoma
- Topical application of natural prostaglandins had been shown to lead to a reduction of IOP. One prostaglandin, PGF2α, had been investigated as a glaucoma treatment and had been found to be effective at lowering IOP. The prodrug PGF2a isopropyl ester (PGF2a-IE) had been found to be a very potent ocular hypotensive with particularly prolonged effects.
- Despite their IOP-lowering activity, natural prostaglandins were not used as anti-glaucoma drugs in the clinic due to their side effects, in particular ocular irritation (pain and so-called “foreign body sensation”) and conjunctival hyperemia (enlarged blood vessels). These side effects had effectively ended any further clinical development of PGF2a-IE as an anti-glaucoma medication.
Animal models
- There were various animal models used for glaucoma. Non-human primates (including monkeys) were the best model, but cats were commonly used to assess IOP lowering. Rabbits were commonly used to test for hyperemia. Cats were commonly used to test for ocular irritation.
The status of prostaglandins as therapeutic agents to treat glaucoma
- There was no authorised prostaglandin drug (natural or analogue) to treat glaucoma. A prostaglandin called latanoprost was a promising candidate for glaucoma treatment having successfully completed Phase II trials and entered Phase III trials.
Classification of prostaglandins and their receptors
- Significant work had been done by 1993 to classify prostaglandins by their target receptors, in particular by Dr R. A. Coleman of Glaxo, which had been published in various books, papers and other publications. Dr Coleman had identified five receptor types labelled DP, EP, FP, IP and TP, which are the targets of the natural prostaglandins PGD2, PGE2¸ PGF2α, PGI2 and TXA2 respectively. The receptor for which PGF2a was the most potent natural ligand was the FP receptor. Dr Coleman also noted tissues thought to be rich in relevant receptors, including that the cat eye iris was rich in FP receptors, and thus the cat iris model was a measure of FP receptor binding.
- The purpose of Dr Coleman’s system was classification and analysis. It was a means for organising information about receptors and ligands and studying them further. It was well-established as a good basis for that kind of action. It was not established as a way of predicting (and certainly not with confidence) that a drug with a particular affinity for prostaglandin receptors would definitely work for a particular therapeutic purpose, still less what its side effects might be, or how they would be mediated.
- Although common general knowledge, the Coleman classification system had limitations and was a work in progress.
Fluprostenol
- Here it is important to quote the judge’s exact findings:
“94. … fluprostenol is mentioned in some of the Coleman classification documents, as a highly potent FP-receptor agonist but which had very low, indeed essentially nil, agonist activity against the other receptor types with which Coleman was concerned. In other words, it was known to be very selective, and indeed more so than PGF2a. This profile made it highly relevant for classification, and so, for example, it is specifically mentioned in the TIPS supplement, and in Dr Coleman’s chapter in Hansch, Comprehensive Medicinal Chemistry (1st Ed, 1990) it is described as ‘diagnostic in establishing whether FP receptors exist in any particular tissue’.
95. So I agree that to this extent and for this purpose, fluprostenol was CGK, as part of the picture concerning the Coleman classification.
96. However, in terms of its previous use as a drug, fluprostenol had merely been a luteolytic agent in veterinary practice, which effect had been attributed to its FP receptor agonism. I do not think this would have been CGK to the skilled addressee working on glaucoma, but even if they had noticed it referred to in passing, it would not have been of practical interest.”
- If fluprostenol was obvious to test or use, so was its isopropyl ester as a prodrug.
The Patent
- The judge summarised the disclosure of the Patent at [99]-[112]. It is unnecessary for the purposes of the appeal to repeat that exercise. It is sufficient to note that it includes data on the effect of FIE (and other compounds) in terms of hyperemia (Example 5) and IOP-lowering (Example 6). The judge set out his assessment of the technical contribution of the Patent at [119]-[121]. The judge accepted Alcon’s case that it had the following aspects:
“i) A new and useful therapy for the treatment of glaucoma …;
ii) A prostaglandin analogue which lowers IOP but without the hyperemia associated with PGF2a;
iii) A prostaglandin analogue which is better than latanoprost in terms of IOP lowering but with comparable good/acceptable hyperemic properties”.
- As the judge noted at [122]:
“It was not argued by Alcon that the technical contribution lies in whole or in part in addressing the side effect of ocular irritation - that is not demonstrated by the Patent for FIE.”
- As is common ground, not only is this not demonstrated by the Patent, but also there are no data at all in the Patent as to the extent to which FIE causes ocular irritation.
The claim
- Claim 1 of the Patent is a claim in the EPC 2000 form for second medical use claims, while claim 2 is a parallel claim in Swiss form. Omitting a disclaimer which is not relevant to the appeal, claim 1 is as follows:
“A topical ophthalmic composition for use in the treatment of glaucoma and ocular hypertension comprising a therapeutically effective amount of fluprostenol isopropyl ester wherein the dosage range for topical administration of fluprostenol isopropyl ester is between 0.05 and 10 mg per eye … wherein fluprostenol isopropyl ester has the following formula:
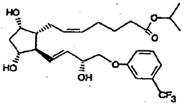 ”
”
Interpretation of the claim
- An issue arose at trial was to whether PGF2α (itself or as the isopropyl ester) was “suitable for use in the treatment of glaucoma” within the meaning of the claim. The judge held that, on the proper interpretation of the claim, this was not the case for the following reasons:
“125. Counsel for the Defendants accepted that there must come a point where side effects are so bad that a drug is not suitable for use despite having an effect on the disease state in question. In particular, he accepted that lethal side effects would prevent a drug being suitable for use. I think that the boundary must depend on context, the disease state including its severity, and the nature and effect of the side effects. So, for example, even the risk of death from side effects might not prevent a cancer chemotherapy from being suitable for use, whereas the same side effects would rule out a drug for, say, bad breath as suitable.
126. In the current context, glaucoma was regarded as an incurable, long-term condition. Neither ocular irritation nor hyperemia would be considered unbearable in the short term, but in the longer term they would be regarded as very burdensome, and were recognised as having a very material adverse effect on patient compliance. They were not just inconveniences.
127. I therefore consider that PGF2a was not suitable for use in treating glaucoma, and I am fortified in that view by the fact that those in the field considered it as being a dead-end, necessitating the search for other agonists with a better side effect profile.”
- It is not in dispute that it follows that, in order to be “suitable for use in the treatment of glaucoma”, FIE must cause lower levels of ocular irritation and/or hyperemia than PGF2α. Aspire contend that FIE must cause both less irritation and less hyperemia, but that does not necessarily follow. As Alcon point out, this was not in issue at trial and so it was not explored in the evidence. I shall return to this point below.
Stjernschantz
- Stjernschantz begins at 691-692 with an introduction which, after a general discussion about earlier misconceptions concerning the role of prostaglandins in inflammation in the eye, and aspects of the available animal models, summarises the evidence concerning the effect of PGF2a and its ester prodrugs in lowering IOP, but with the side effects of irritation and hyperemia. The introduction concludes by summarising earlier work by the authors:
“However, substituting part of the omega chain with a phenyl ring (Scheme 2) has been shown to change the pharmaceutical profile of PGF2α dramatically with respect to the side effects in the eye (26)-(29).”
- From 692 to 695 there is a long section describing a general method for synthesis of phenyl substituted PGF2a analogues. This is not relevant for present purposes, save to note that Scheme 2 shows seven 17-phenyl substituted PGF2a-IE analogues in which latanoprost is compound 8, and its racemate is compound 11:
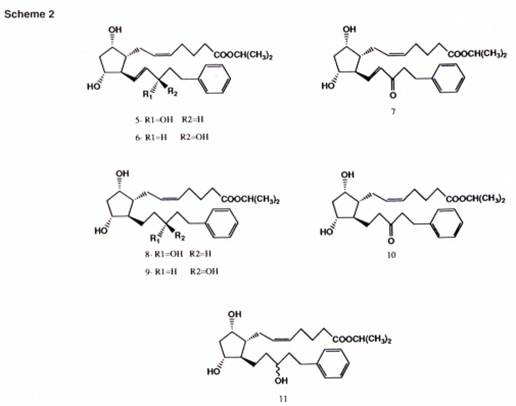
- From 695 to 698 Stjernschantz analyses the structure-activity relationships (SARs) of the Scheme 2 compounds. As shown by Figure 2, they all had marked and dose-dependent miotic (pupillary constrictive) effect in a cat eye model. (Later, at 700, the authors explain that “the miotic effect in cats expressing a FP receptor function seems to correlate with the IOP reducing effect in primates and man”.) Table I sets out their irritative and hyperemic effect in the cat’s eye, showing no irritation, but some hyperemia, albeit less than that exhibited by PGF2a-IE. The authors comment:
“The marked miotic effect of these compounds in combination with the total lack of irritative effect strongly suggests that substitution of part of the omega chain with an aromatic ring structure either causes conformational alteration in the molecule or imposes a steric hindrance, which enables a discrimination between different prostaglandin receptor subtypes.”
- The IOP effect of the Scheme 2 compounds was tested in monkeys, with the results shown in Figure 3, where compounds 5, 8 and 11 were particularly effective and roughly equipotent with PGF2a-IE.
- At the end of this section, at 698, the authors state:
“As can be seen in Table I, all phenyl substituted PGF2alpha-ie analogues (Scheme 2) induced clearly less hyperemia than PGF2alpha-ie. The analogues exhibiting least conjunctival hyperemia were generally those exhibiting least pharmacologic activity such as the earlier mentioned 15-OH epimers 6, 9 and the 15-keto 7, 10 17-phenyl substituted prostaglandin analogues (Table I).”
- From 698 to 699 Stjernschantz discusses latanoprost, “[a] new drug candidate for glaucoma treatment”. It explains at 698-699:
“The prostaglandin receptor profile of latanoprost has been worked out in vitro using a receptor classification system previously described (56). … Latanoprost has high affinity and selectivity for PGF2α (FP) receptors as demonstrated in Table III (57). The affinity for EP2, EP3, DP, IP and TP receptors is very low compared with the specific ligands [i.e. PGE2 etc]. However, the affinity for EP1 receptors is somewhat greater (57). These results indicate that FP receptors most likely are important in the mechanism leading to increased uveoscleral outflow and reduced IOP in primate and human eyes.”
- Reference 56 is Dr Coleman and his co-authors’ chapter in Hansch et al, Comprehensive Medicinal Chemistry. The judge held that, even if the skilled team did not know about the Coleman classification as a matter of common general knowledge, they would look this reference up so as to be able to understand this part of the teaching of Stjernschantz. Reference 57 is a previous paper by Stjernschantz, Resul and two co-workers.
- At 699 the authors summarise the existing clinical trial data for latanoprost as follows:
“… all clinical studies performed so far indicate that latanoprost (including PhXA34 [its racemate]) effectively reduces IOP with markedly improved side effect profile compared to PGF2α and its isopropyl ester.”
- From 700 to 701 Stjernschantz considers the effect of varying the length of the phenyl substituted omega chain (the chain at the bottom of each of the structures shown in Scheme 2) from 3 carbons to 12 carbons (from C-15 phenyl substituted to C-24 phenyl substituted). Figure 4 shows the miotic and irritative effects of these analogues. Compound 5, the 17-phenyl substituted analogue, was found to be optimal, exhibiting “high biological activity without irritating effect”.
- At 700 the authors comment:
“These results indicate that the 17-phenyl-18,19,20-trinor- PGF2alpha-ie is unique in that this compound exhibits a structural conformation with no affinity for PG receptors involved in the sensory irritative response (presumably PG receptors on sensory nerves), while retaining the affinity for FP receptors as demonstrated by the miotic response. In contrast, PGF2alpha-ie analogues with shorter or longer phenyl substituted omega chain did show some affinity for PG receptors mediating nociceptive impulses. However, this affinity was much weaker than that of PGF2alpha-ie. It appears that the steric hindrance of the phenyl ring and the interatomic distances between functional groups in the molecules are important for drug-receptor interaction.”
- At 701 Stjernschantz considers the effect of introducing substituents into the phenyl ring, specifically a methyl group, methoxy group, trifluoromethyl group or fluorine, as shown in Scheme 8. Some of these groups did not change the miotic activity, whereas others did. In particular:
“The introduction of a trifluoromethyl group into position 4 in the phenyl ring 23, as can be expected, rendered the 17-phenyl-18,19,20-trinor-PGF2alpha-ie analogue practically inactive (Table IV).”
- From 701 to 702 Stjernschantz comments on the “[i]mportance of ring structure on the omega chain” as follows:
“From what is mentioned above, it is evident that by substituting part of the omega chain of PGF2alpha-ie with a phenyl ring, it is possible to totally eliminate the ocular irritating effect and to markedly reduce the hyperemic effect of PGF2alpha-ie. Although a phenyl ring substitution seems to be particularly beneficial, substitution with other ring structures such as cyclohexyl, thiophene and biphenyl also yields compounds with distinctly better side effect profile than that of PGF2alpha and its prodrugs in the eye (unpublished results). Thus, PGF2alpha possessing a terminal ring moiety on the omega chain exhibits a markedly improved therapeutic index in the eye.”
- After explaining that the authors have studied several other phenyl substituted prostaglandins, Stjernschantz comments at 702:
“It is obvious that similar phenyl substitutions e.g. of PGB2, PGC2 or PGD2 can be anticipated analogously to improve the side effect profile of these prostaglandin analogues in the eye.”
- Stjernschantz sets out the authors’ conclusions at 702 as follows:
“PGF2alpha and its isopropyl ester have been shown to be potent ocular hypotensive agents in several animal species and in man. However, the frequent and disturbing side effects in the eye make it impossible to utilize PGF2alpha as an ocular hypotensive agent clinically. Whereas the prodrug esters of PGF2alpha do not significantly reduce the adverse effects in the eye, partial substitution of the omega chain with a phenyl ring dramatically reduces the ocular side effects of PGF2alpha-ie. Such substitution totally eliminates the superficial irritating effect of PGF2alpha-ie in the eye. This is probably due to a conformational change of the omega chain in the prostaglandin molecule, or steric hindrance, which enables a discrimination between different prostaglandin receptor subtypes. The most optimal chain length to which the ring structure is attached seems to be 5 carbon atoms (17-phenyl-18,19,20-trinor). The biologic activity of these compounds may further be altered by substitutions in the phenyl ring.
One of the most promising analogues 8 latanoprost is presently undergoing phase II clinical testing with encouraging results. This drug has been shown to potently reduce IOP in glaucoma patients with few side effects.”
Obviousness
The Defendants’ case
- As the judge explained at [172]:
“The Defendants’ case was that Stjernschantz showed that it was most probably FP receptor binding that was responsible for reduced IOP, while side-effects were mediated by other prostaglandin receptors, and that once that was known, it would be obvious to try FIE for treating glaucoma, because it was known from the CGK to be a potent and selective FP receptor agonist. Thus, they argued, FIE fit the profile of an efficacious, side-effect sparing prostaglandin analogue.”
As the judge noted at [175], this case “is a simple one”.
The judge’s assessment
- The judge’s assessment was as follows. He began by addressing Alcon’s argument that the Defendants’ case was really an attack based on the common general knowledge alone:
“176. I disagree with Alcon on this; the Defendants were clearly building their attack over Stjernschantz, which contained what was an important building-block in their argument, namely the concrete identification of the FP receptor’s involvement. However, I agree with Alcon in a more general sense that the Defendants’ attack was developed at a considerably greater level of simplicity and generality than either Stjernschantz or the CGK justified, and did not address adequately a number of more detailed, practical points.
177. I think the following factors are particularly important:
i) The field, as I have identified it in addressing the skilled addressee was at a very early stage and there were many uncertainties in it.
ii) The field had hit a barrier with the failure of PGF2a because of side effects.
iii) The field was just starting to recover from that with the development of latanoprost.
iv) Stjernschantz reports encouraging early results with latanoprost, but solid data was still awaited.
v) Stjernschantz reports a theory about the cause of side effects but without proof; Stjernschantz focuses on prostaglandins and their receptors, but does not contain a demonstration that the side effects experienced were a result of action at prostaglandin receptors alone.
vi) Although Stjernschantz’s results in relation to the side effect of irritation were very good, the position on hyperemia was much less clear and there was an indication that the better the effect on IOP the greater the hyperemia. It was hard to see how to progress from there.
178. These issues all would mean that once the skilled addressee looked into the detail of Stjernschantz, their confidence that progress could be made with improving or maintaining IOP reduction while reducing hyperemia would be very limited.”
- Having noted that Stjernschantz included aspects of pharmacology and aspects of medicinal chemistry, both of which were important, and that it did not mention fluprostenol at all, the judge went on:
“181. In my view, the natural way for the skilled team to approach obvious developments from Stjernschantz would be to consider further prostaglandin analogues, altered in ways concretely reasoned out from the structure activity work described. This would be logical and routine and in keeping with the approach of the paper. It is suggested in the penultimate paragraph of the paper.
…
183. I must not overlook that more than one approach may be obvious from a piece of prior art. In principle it could be obvious both to make structural changes based on Stjernschantz’s structure activity relationship work and also obvious to work with compounds with significantly different structures but similar patterns of activity. It depends on the facts, but Stjernschantz’s being a paper with such a strong basis in structure activity relationships is a point against trying other compounds based on activity.”
- Having noted that it was clearly the Defendants’ case that FIE was obvious to try based on its affinity and selectivity for the FP receptor and not structure, the judge went on:
“186. In my view, the Defendants’ attack has the following significant problems:
i) The CGK of fluprostenol was as an analytical tool, not a medicine.
ii) Insofar as fluprostenol was known as a medicine, it had achieved use only in a very different field (luteolysis in animals).
iii) Fluprostenol is not mentioned in Stjernschantz ….
iv) No case was made that fluprostenol would be specifically identified as consistent with the structure activity relationship work in Stjernschantz.
v) Efforts with fluprostenol might be accompanied by the hope that it would give better IOP lowering and/or reduced side effects, but there could be no positive expectation. And in particular, it would be thought, for reasons explained above, that a better IOP lowering effect would be accompanied by more hyperemia.
187. The last point bears some expansion: motivation and expectation of success may be important factors when it comes to obviousness, and their relative importance depends on the context and the facts, but a hope for a positive result sufficient to justify research being done does not necessarily imply an expectation of success …. Even had the skilled team thought of trying fluprostenol in animal models to assess its possible use to treat glaucoma, they would have regarded it as very uncertain what effect it would have on side effects. Even if they had thought (which I do not think was established) that initial animal experiments with fluprostenol would have needed such low resources that it could be justified as a gamble, that would not make it obvious.
188. But in any event, bearing points i) to iii) in mind in the context of the overall direction of Stjernschantz, I do not think the skilled team would without invention have turned its mind to fluprostenol as a possible treatment in the first place. Even if it did, the prospects of success (having regard to efficacy and side effects) would be very uncertain. Much more attractive options consistent with the overall teaching and direction of Stjernschantz were available.”
- Finally, the judge turned to consider the evidence relied upon by the Defendants. He did not find Dr Wilson’s evidence persuasive for the reasons he gave at [191]:
“… In particular, I thought it failed to deal with why the skilled team would think of fluprostenol in the first place, was almost entirely lacking in analysis of the prospects of success to be expected, and did not take account of the nature of the work done, and suggestions by, Stjernschantz.”
- As for Dr Redshaw’s evidence, the judge commented at [193]:
“I found her analysis very limited and not a good basis for the proposition that the skilled addressee would just switch to other, quite different compounds. I did not think it properly reflected how a medicinal chemist would approach Stjernschantz; they would read the whole of it carefully and suggest specific, rational, structural changes based on the SAR work done.”
- The Defendants also relied upon certain answers given by Dr Krauss and Dr Cavalla in cross-examination, but the judge said at [194]:
“I think it is particularly important in the present case to read the whole passages. When that is done, I think the answers given were not really supportive of the Defendants’ case, and certainly not enough to undermine the evidence that Alcon’s experts had given in their written reports, which in general they continued to support and which I preferred over that of the Defendants’ experts.”
The appeal
- Obviousness involves a multi-factorial evaluation and therefore this Court is not justified in intervening in the absence of an error of law or principle on the part of the judge. Aspire contends that the judge erred in principle in three main respects: first, as noted above, he omitted to make a finding as to the relationship between the members of the skilled team; secondly, he misinterpreted Stjernschantz; and thirdly, he made findings which were not open to him on the expert evidence.
- The relationship between the members of the skilled team. Aspire contends that the judge ought to have found that the skilled team would be led by the pharmacologist.
- In response to this contention, counsel for Alcon cited what Jacob LJ giving the judgment of the Court of Appeal said in Halliburton Energy Services Inc v Smith International (North Sea) Ltd [2006] EWCA Civ 1715 at [14]:
“Mr Burkill, in his skeleton argument, advanced an argument to the effect that where the skilled addressee was taken to be a team (because more than one skill was involved) then the law requires one member of the team to be the “head” directing the others. Here the suggestion was that the head of the team would be a rock bit engineer who would be directing a computer model designer as some kind of assistant. That position was not pursued during the oral argument. And rightly so. If the addressee of a patent is a notional team of persons with differing skills, then it is a team with no boss. Each member of the team is assumed to play his/her own part.”
- As counsel for Aspire pointed out, the submission which Jacob LJ rejected was that the law requires one member of the team to be the head of the team directing the other(s). As counsel for Alcon rightly accepted during the course of argument, it would be equally incorrect to say that the law requires the team to have no leader. As both counsel agreed, the important point made by Jacob LJ is that each member of the team is assumed to play their own part. The nature of each member’s role, and the relationship between them, is inevitably fact-specific. In some cases, this may involve one member taking the lead: see e.g. KCI Licensing Inc v Smith & Nephew plc [2010] EWHC 1487 (Pat), [2010] FSR 31 at [103] and Generics (UK) Ltd v Warner-Lambert Co LLC [2015] EWHC 2548 (Pat), [2016] RPC 3 at [118]-[119].
- In the present case Dr Krauss, Dr Cavalla and Dr Redshaw all stated in terms in their reports that the skilled team would be led by the pharmacologist. Furthermore, Dr Cavalla and Dr Redshaw were agreed that a key reason for this was that the medicinal chemist would have no special knowledge of either glaucoma or prostaglandins, but rather would be a generalist. Accordingly, as Dr Krauss explained in his first report (Dr Cavalla’s evidence was to the same effect):
“19. … The skilled pharmacologist considering new treatments for glaucoma would be responsible for identifying a potential biological target or pathway which could be pursued … The skilled pharmacologist would then, with the help of the skilled medicinal chemist, develop potential compounds which act on the biological target/pathway. The skilled pharmacologist would then test the activity of these potential compounds … by designing and performing in vitro and in vivo assays. …
20. The skilled medicinal chemist would be led by the skilled pharmacologist and be responsible for synthesising drug candidates for the skilled pharmacologist to test. They would also work with the skilled pharmacologist in assessing how the structure of the potential drug candidate compounds may influence their pharmacological action and therefore in designing potential drug candidates. This would be likely be a trial and error process in which potential drug candidates are made and their activity tested, further structural alterations are made to the … candidates based on the results, and then the modified drugs are, in turn, tested to assess the effect of the modification.”
- That being the state of the evidence, I agree with Aspire that the judge should have found as much. Counsel for Aspire rightly did not suggest, however, that this in itself undermined the judge’s assessment of obviousness. Rather, she submitted that it fed into his error in assessing the expert evidence. I shall consider that alleged error below.
- Interpretation of Stjernschantz. Aspire contends that the judge made two errors in his interpretation of Stjernschantz.
- First, Aspire submits that the judge misinterpreted the second sentence in the passage at 698 quoted in paragraph 37 above. For convenience I will set out the sentence in question again:
“The analogues exhibiting least conjunctival hyperemia were generally those exhibiting least pharmacologic activity such as the earlier mentioned 15-OH epimers 6, 9 and the 15-keto 7, 10 17-phenyl substituted prostaglandin analogues (Table I).”
- The judge said at [152]:
“I agree with Alcon that this gives the impression that hyperemia was in some way correlated to activity. It would reduce any confidence that the skilled addressee could otherwise have that it would be possible to achieve good activity (matching latanoprost, for example), while reducing or eliminating hyperemia.”
- As counsel for Aspire pointed out, Dr Krauss quoted this sentence from Stjernschantz in his first report at paragraph 136, but commented in paragraph 137 that “looking at the data the skilled pharmacologist would conclude that there was no clear correlation between the observed hyperemia in the different analogues tested and their ‘pharmacological activity’ in the cat miosis and normotensive studies”. Having ranked the results from each study in his Table 1, Dr Krauss explained in paragraph 138:
“… there is reasonably good correlation between the mioisis and IOP-lowering data at the same dose (1.0 μg). However, based on the lack of correlation between miosis, IOP-lowering and hyperemia, the skilled pharmacologist would conclude that the biological mechanism for the hyperemia is unclear and it cannot be said that those with the lowest efficacy have the lowest hyperemia (see for example compound 11 which ranks highly for cat miosis and IOP-lowering and causes some of the lowest levels of hyperemia).”
- Based on this unchallenged evidence from Alcon’s own pharmacology expert, counsel for Aspire submitted that the judge’s reading of this sentence was wrong. The significance of this, counsel argued, was that this misreading of Stjernschantz was the foundation for what the judge went on to say at [177(vi)], [186(v)] and [187].
- I do not accept this submission. What the judge said was that the sentence “gives the impression that hyperemia was in some way correlated to activity [emphasis added]”. As a reading of the text of Stjernschantz that is unimpeachable. Moreover, it is supported by evidence given by both Dr Wilson and Dr Redshaw in cross-examination that the sentence suggests that there appears to be a correlation between hyperemia and pharmacological activity.
- It is fair to say that the evidence of Dr Krauss indicates that the pharmacologist would be likely to conclude that the data did not support the statement in the text, even bearing in mind the authors’ attempt to cover themselves by their use of the word “generally”. This does not assist Aspire, however. In the first place, the judge’s reading of this sentence of Stjernschantz was as only conveying a tentative message, not a robust conclusion. Secondly, and more fundamentally, the key point in Dr Krauss’ evidence was that “based on the lack of correlation between miosis, IOP-lowering and hyperemia, the skilled pharmacologist would conclude that the biological mechanism for the hyperemia is unclear”. Thus the data would not have given the skilled team any confidence that they could maintain or increase IOP-lowering while reducing or eliminating hyperemia by using a different analogue to those studied in Stjernschantz.
- Secondly, Aspire contends that the judge misinterpreted the final sentence of the penultimate paragraph of Stjernschantz’s conclusions at 702. I have quoted the conclusions in full in paragraph 46 above, but for the convenience I will set out the sentence in question again:
“The biologic activity of these compounds may further be altered by substitutions in the phenyl ring.”
- Aspire criticises the judge’s statement at [181] (quoted in full in paragraph 47 above) that consideration of “further prostaglandin analogues, altered in ways concretely reasoned out from the structure active work described” is “suggested in the penultimate paragraph of the paper”.
- Counsel for Aspire submitted that this is wrong, and that what the sentence I have quoted in paragraph 67 is referring to is the work described by the authors at 701-702 (see paragraph 41 above). The significance of this, counsel argued, was that this misreading of Stjernschantz was a key plank in the judge’s rejection of the Defendants’ case, as could be seen not only from what he said at [181], but also from what he went on to say in [183], [186(iv)], [188] and [193].
- Again, I do not accept this submission. I agree that the sentence in question primarily refers back to the work described by the authors at 701-702. As the medical chemist would appreciate, however, that work was not exhaustive: the authors only studied four substituents in a limited number of positions on the phenyl ring. Stjernschantz leaves plenty of scope for further investigations based on that SAR work using different substituents, ring positions and combinations thereof. The sentence in question embraces such further investigations. Furthermore, the statement in the preceding sentence that “[t]he most optimal chain length to which the ring structure is attached seems to be 5 carbon atoms (17-phenyl-18,19,20-trinor) [emphasis added]” indicates the potential for further research based on the authors’ work on varying the chain length described at 700-701 (see paragraph 41 above). Yet further, the work reported on other ring structures mentioned at 702 (quoted in paragraph 44 above) indicates that alternatives to a phenyl ring are worth exploring, while the statement at 702 that “[i]t is obvious that similar phenyl substitutions” of other prostaglandins “can be anticipated analogously to improve the side effect profile” (see paragraph 45 above) points to a fourth avenue of structural variation. Finally, although the text of Stjernschantz does not draw attention to these aspects, the medicinal chemist would note from Scheme 2 that the work reported included two more types of structural variation, both of which would be susceptible to further investigation: (i) the bond between the carbon atoms in positions 13 and 14 (which is a double bond in compounds 5, 6 and 7, but a single bond in compounds 8-11); and (ii) the moiety at position 15 (which is carbon-hydroxyl (C-OH) in compounds 5-6 (with alternative stereochemistries), 8-9 (with alternative stereochemistries) and 11 (the racemate of 8 and 9) but keto (C=O) in compounds 7 and 10).
- Not only is this clear from Stjernschantz, but also it is supported by the evidence of Dr Cavalla which the judge was entitled to and did accept. As Dr Cavalla put it in cross-examination, “[to] a medicinal chemist, their bread and butter is structural variation”. Having surveyed the SAR work described in Stjernschantz, Dr Cavalla set out in paragraph 121 of his first report a list of potential further structural variations of compounds 5, 8 (latanoprost) and 11 in Stjernschantz, including those I have discussed in the preceding paragraph. Dr Redshaw gave no evidence to the contrary, because, as the judge explained, she had been led by her instructions not fully to consider the medicinal chemistry aspects of Stjernschantz.
- Accordingly, the judge was correct - or at least fully entitled - to conclude that the skilled team reading Stjernschantz would understand it to be suggesting the investigation of “further prostaglandin analogues, altered in ways concretely reasoned out from the structure active work described”.
- The expert evidence. Aspire advanced two principal, somewhat contradictory criticisms, of the judge’s assessment of the expert evidence.
- First, in its skeleton argument Aspire contended that the judge was not entitled to find that it was not obvious to try fluprostenol in the light of Stjernschantz because the evidence of Dr Krauss was predicated upon the pharmacologist not knowing about prostaglandins, and thus not having much of the common general knowledge which the judge found that they would have, and in particular the knowledge of fluprostenol. Counsel for Aspire did not in the end pursue this contention in her oral submissions: as the judge noted at [18], and counsel accepted, in his second report Dr Krauss did address the issue from the perspective of the prostaglandin specialist. (Counsel for Aspire did submit that, in that report, Dr Krauss had not considered the correct question; but that is a different point, and I do not accept the submission anyway.)
- Secondly, both in its skeleton argument and counsel’s oral submissions, Aspire contended that the judge was not entitled to find that the obvious path from Stjernschantz was to consider further prostaglandin analogues, reasoned out from the structure activity work described in Stjernschantz, because this was contrary to the evidence, and in particular the evidence of Dr Krauss.
- Counsel for Aspire argued that this was where the judge’s omission to find that the skilled team would be led by the pharmacologist became significant. Because the team would be led by the pharmacologist, she submitted, it would be the pharmacologist who would decide what direction the team should take after reading Stjernschantz. Dr Krauss’s evidence in his first report was, she submitted, unequivocal:
“234. While they may discuss the possibility with the skilled medicinal chemist, I also do not believe that the skilled pharmacologist would consider investigating other phenyl-substituted analogues of PGF2a. This is as the skilled pharmacologist would have no reason to believe that it would be possible that structural modifications to compound 5, latanoprost and/or compound 11 would result in a better drug.
…
239. I therefore do not believe the skilled pharmacologist would be motivated to investigate other phenyl-substituted analogues and, if they were, it would be a lengthy research program to investigate the potential modifications that could be made.”
- Counsel for Aspire further submitted that the judge’s finding was not merely contrary to Dr Krauss’ evidence, but also contrary to, or at least unsupported by, the other expert evidence. In this connection she relied upon the following evidence:
i) The evidence of Dr Wilson in paragraphs 141-144 of his first report that it would be obvious to the pharmacologist in the light of Stjernschantz “to test other potent and selective FP agonists as alternative compounds for use in the treatment of glaucoma”, that “fluprostenol was the most potent and selective FP agonist available (or at least one of the best available)” and therefore that “fluprostenol would be the first compound to be tested”.
ii) The evidence of Dr Redshaw in paragraphs 70-72 of her first report that she agreed with Dr Wilson; that she had been asked whether the skilled team, and in particular the medicinal chemist, would wish to synthesise further compounds within the series of 17-phenyl substituted PGF2α analogues; and that, in her opinion, “the most straightforward approach, and that most likely to lead to early success, would have been to test known PGF2α analogues that had been reported to have, like latanoprost, high affinity for the FP receptor and good selectivity …”.
iii) The evidence of Dr Cavalla in paragraph 118 of his first report:
“The skilled team would be guided by the skilled pharmacologist as to whether it would be worthwhile to take any further steps after reading Stjernschantz. This is as the skilled team would only consider further work if the skilled pharmacologist thought any of the compounds in Stjernschantz were sufficiently interesting for further development and I would defer to Dr Krauss in this respect. I understand from Bristows that it is Dr Krauss’ evidence that there are a number of potential steps that the skilled pharmacologist may take after reading Stjernschantz, one of which would be to discuss with the skilled medicinal chemist whether it would be possible to modify compounds 5, 8 or 11 to either: (i) improve the efficacy while maintaining (or ideally lowering) any side effects; or (ii) maintaining (or ideally improving) the efficacy while improving the side effects.”
- Persuasively though they were advanced by counsel for Aspire, I do not accept these submissions for the following reasons.
- Although I have accepted that the evidence established that the skilled team would be led by the pharmacologist, as counsel for Alcon pointed out, it does not follow that the medicinal chemist would have no role in deciding what steps to take in the light of Stjernschantz. On the contrary, the evidence shows that, as one would expect, this would be a matter for discussion between the two members of the skilled team. Thus the input of the medicinal chemist cannot be ignored.
- So far as Dr Krauss’ evidence is concerned, while read in isolation paragraphs 234 and 239 may appear to support the submission based on them, they must be read in context. In context what Dr Krauss was saying was that: (i) the pharmacologist would consider latanoprost (compound 8) and compound 11 as worth taking forward as potential new treatments for glaucoma (paragraph 231): (ii) the pharmacologist might also consider further developing compound 5 (paragraph 232); (iii) while the pharmacologist might discuss the possibility of investigating structural modifications to compounds 5, 8 or 11 with the medicinal chemist, they would have no reason to believe that this would result in a better drug (paragraph 234); (iv) investigating other phenyl substituted analogues would take a research programme with no expectation that this would be fruitful (paragraph 236), in particular because there are no data in Stjernschantz which indicate how it would be possible to increase the efficacy or reduce the side effects of compounds 5, 8 or 11 (paragraphs 237 and 238); (v) accordingly the pharmacologist would not be motivated to investigate other phenyl substituted analogues, but if they were it would be a lengthy research program (paragraph 239); and (vi) the other potential modifications discussed in Stjernschantz would present other alternative research avenues for investigation (paragraph 240). Thus Dr Krauss was not disputing that Stjernschantz suggested various possible structural variations which could be investigated, he was saying that the pharmacologist would not be motivated to go down that road because they would not have any expectation of success and it would require a lengthy research project.
- Turning to Dr Wilson, he did not opine in his report on whether or not it was obvious in the light of Stjernschantz to consider further prostaglandin analogues, reasoned out from the structure activity work described there. He did give his opinion as to the obviousness of trying fluprostenol; but that evidence did not exclude the possibility that structural variation was an obvious course to adopt.
- As for Dr Cavalla, it is important to take into account what he said in his second report. At paragraph 35 he repeated the point he had made in paragraph 164 of his first report that there are three structural differences between latanoprost and FIE, as shown below.
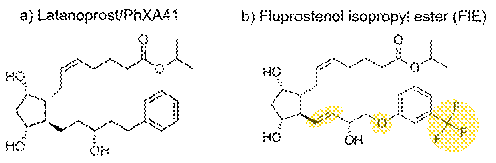
- In paragraph 36 Dr Cavalla said that there was no definitive way for the medicinal chemist to predict how these differences would affect the pharmacological profile of fluprostenol and its suitability for the treatment of glaucoma, but the structure-activity data disclosed in Stjernschantz would suggest the following: (i) substitution of the phenyl ring with a trifluoromethyl group would be predicted to make fluprostenol less efficacious at lowering IOP than latanoprost; (ii) the double bond between carbons 13 and 14 might increase hyperemia; (iii) replacing the carbon at position 17 with an oxygen would be likely to result in hydrogen-bonding between the hydroxyl group at carbon 15 and the oxygen that could disrupt the function of the hydroxyl group, which was important to the pharmacological activity of latanoprost.
- Dr Cavalla said in paragraphs 37 and 38 that the medicinal chemist would conclude that it was unlikely that fluprostenol would have the same pharmacological profile as latanoprost, and therefore he disagreed with the opinion expressed by Dr Redshaw in paragraph 72 of her first report as to the “most straightforward approach”. He went on in paragraph 39:
“Instead, the ‘most straightforward approach’ to the … medicinal chemist would be to make routine modifications to the structure of latanoprost to improve its pharmacological effects. However … the … medicinal chemist would have no way of knowing how they would affect the pharmacological properties observed in latanoprost. The … medicinal chemist would also assume that … Stjernschantz had already tested a number of different modifications to latanoprost and alighted upon latanoprost as the best. The … medicinal chemist is therefore left with many different potential modifications to try with no reason to predict that they would improve the pharmacological properties of latanoprost.”
- Dr Cavalla did not resile from this evidence in cross-examination, although he agreed that “looking backwards” testing fluprostenol “may seem to be an obvious thing to do”. The furthest he went was to accept that fluprostenol would “possibly” be on the list of compounds to test. Furthermore, Dr Cavalla explained that, even if the medicinal chemist had addressed their mind to selecting an FP agonist, the question would remain as to how to get the same or better efficacy as latanoprost with reduced or the same side effects.
- So far as Dr Redshaw is concerned, as the judge explained and I have noted already, the problem with her evidence was that, despite being the medicinal chemist on the team, she had not fully or properly considered the medicinal chemistry aspect of Stjernschantz.
- I therefore consider that, on the evidence, the judge was fully entitled to conclude that the obvious way forward from Stjernschantz was “to consider further prostaglandin analogues, altered in ways concretely reasoned out from the structure activity work described”.
- As the judge correctly recognised at [183], that did not necessarily exclude the possibility that another step might also be obvious. He went on carefully to consider the Defendants’ case that it would be obvious to try fluprostenol, and gave cogent reasons for rejecting it. As he explained at [188], one of his reasons was that, having regard to the points made at [186(i)-(iii)], he did not think that the skilled team would without invention have turned its mind to fluprostenol as a possible treatment in the first place. This ties in with his explanation at [191] that one of the reasons he did not find Dr Wilson’s evidence persuasive was that it failed to deal with why the skilled team would think of fluprostenol (i.e. given that it was only known for its use as an analytical tool and was not known to have any relevant therapeutic effect). In other words, although the judge did not put it this way, Dr Wilson’s evidence was tainted by hindsight.
- A final point which I should mention is that counsel for Aspire submitted that the judge had been wrong to find that the skilled team would have little or no expectation of success if they were to try fluprostenol. This submission was predicated upon the grounds of appeal which I have already rejected, however. It was not advanced as a free-standing ground of appeal, no doubt because counsel recognised that it could not be suggested that the judge had made any other error of principle in making that finding. In any event, the point does not arise given the judge’s finding that the skilled team would not even think of fluprostenol.
Insufficiency
The law
- Since the Patent is a patent for a second medical use of a known medicinal compound, the Patent must plausibly disclose the effect that it claims. The criterion for plausibility is stated by Lord Sumption in Warner-Lambert Co LLC v Generics (UK) Ltd [2018] UKSC 56, [2019] Bus LR 360 at [36]: “the specification must disclose some reason for supposing that the implied assertion of efficacy in the claim is true”.
The Defendants’ case
- Like the judge, I shall quote the Defendants’ case as it was pleaded (as amended at trial):
“In the event that the claims of the Patent are not obvious because it was understood that the administration of PGF2α isopropyl ester would cause ocular inflammationhyperemia and irritation, the Patent fails to make plausible that the compounds of the invention are suitable for use in treating glaucoma and ocular hypertension. In particular, the data in the specification of the Patent fails to evidence an improvement in these side effects when the compounds of the invention are used over PGF2α isopropyl ester.”
- As is common ground, this is a so-called “squeeze” argument taking the logical form “if … then …”. The point of such an argument is to prevent the patentee from relying upon an effect that is not plausibly disclosed by the patent to defeat an argument of obviousness.
The judge’s assessment
- The judge rejected the Defendants’ case for the reason he gave at [199]:
“The point fails on the facts … since, as I have explained above, the Patent does make it plausible that FIE causes reduced hyperemia compared to PGF2α.”
The appeal
- Aspire contends that the judge’s reason discloses a plain error of principle: although it followed from the judge’s interpretation of the claim that, in order to be “suitable for use in the treatment of glaucoma”, FIE should cause lower levels of both irritation and glaucoma than PGF2α, he only found that the Patent plausibly disclosed reduced hyperemia. He made no such finding with respect to irritation: on the contrary, he correctly found that the Patent did not plausibly disclose reduced irritation.
- As Aspire points out, when Aspire sought permission to appeal on this ground, the judge rather surprisingly refused permission because “irritation was not run as part of [Alcon’s] technical contribution”. As counsel for Aspire submitted, and counsel for Alcon did not contest, this is legally erroneous: the technical contribution of a patent is not a matter which depends on the patentee’s case, it is an objective question for determination by the court in the light of all the relevant evidence. It follows that it is no answer to this ground of appeal that Alcon did not claim that lower irritation was part of the technical contribution of the Patent. To the contrary, that supports Aspire’s position.
- It does not follow, however, that the judge was wrong to reject the Defendants’ case. As Alcon point out, the pleaded case was not a free-standing attack of lack of plausible disclosure of reduced irritation. If such a case had been pleaded, Alcon contend that they could and would have adduced evidence to meet it. This would have involved addressing the issue of claim interpretation referred to in paragraph 32 above, and exploring the extent to which irritation may be tolerated if hyperemia is reduced. As it was, Dr Krauss gave unchallenged evidence that timolol, the first line treatment in 1993, caused irritation, so it is clear that some level of irritation was acceptable.
- Instead, as discussed above, the pleaded case is purely a squeeze. As Alcon point out, the judge did not reject the Defendants’ case of obviousness “because it was understood that the administration of PGF2α isopropyl ester would cause ocular … irritation”. Nor, perhaps more pertinently, did he reject it because FIE caused a lower level of irritation than PGF2a. On the contrary, he rejected the Defendants’ case of obviousness for quite different reasons, as discussed above. Thus the premise for the insufficiency argument to apply did not arise.
Conclusion
- For the reasons given above I would dismiss this appeal.
Nugee LJ:
- I agree.
King LJ:
- I also agree.

 ”
”